Mike Rice, Beth Fordham-Meier, Andrew Ng
Cello Health BioConsulting, now part of Lumanity
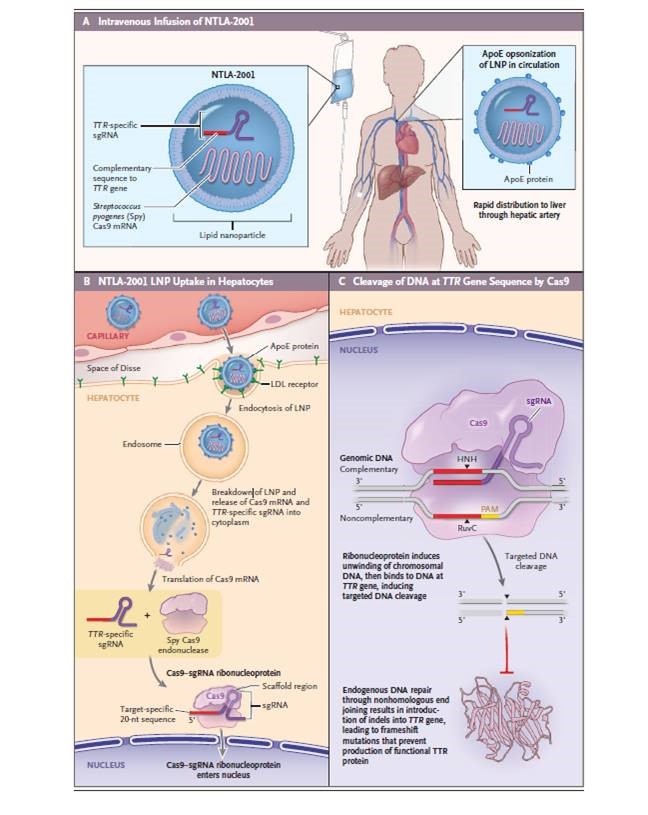
Amidst success of in vivo adeno-associated virus (AAV) based gene therapies, the gene therapy community awaits key outcomes from next month’s Cellular, Tissue and Gene Therapies Advisory Committee (CTGTAC) meeting discussing toxicities and deaths associated with high titer AAV dosing and potential long-term adverse events. While this discussion evolves, a significant advance for in vivo non-viral gene editing was published in a landmark report by Intellia and Regeneron demonstrating, for the first time, the safety and efficacy of non-viral, lipid nanoparticle (LNP) administered CRISPR-Cas9 gene editing in the human liver. The report demonstrated that NTLA-2001 was efficiently delivered to the liver and knocked out the TTR gene in hepatocytes with a single dose treatment. Accumulations of misfolded transthyretin in systemic tissues is responsible for Amyloidosis (ATTR), which presents clinically with polyneuropathy and/or cardiomyopathy. While chronically administered drugs (tafamidis/Vyndaqel, inotersen/Tegsedi and patisiran/Onpattro) for ATTR have recently been developed, one-time treatment of NTLA-2001 has the potential for lifelong reduction of TTR protein and reversing disease progression.
This is especially significant when considering that, back in 2014 when Intellia was being founded, there were serious, perhaps existentialist questions around the feasibility of in vivo delivery of gene editing payloads. In that climate of heightened scrutiny around the safety and ethics of gene therapy, innovators and investors wondered whether it would be possible to achieve therapeutically relevant gene editing efficiency with a safety package sufficient to satisfy regulators. In fact, shortly after founding Intellia, in a move designed to mitigate some in vivo delivery and safety (e.g. immunogenicity and off-target cleavage) risks, the firm launched its eXtellia division focused on ex-vivo modified hematopoietic stem cells for Sickle Cell Disease and adoptive cellular therapies (ACT) for cancer and autoimmune diseases. However, since then, the FDA has become more favorable to novel therapeutic approaches and reviews gene therapies as it would any other drug. In fact, the FDA provides an expedited development pathway through the Regenerative Medicine Advanced Therapy Designation (RMAT), which became available through the 21st Century Cures Act.
Going forward, similarly as N-Acetylgalactosamine (GalNAc) conjugated oligonucleotides capable of targeting hepatocytes has opened the liver as a therapeutic target for a variety of antisense oligonucleotide (ASO) and small interfering RNAs (siRNA) based therapeutics, Intellia has opened the liver as a platform to develop stable in-vivo gene editing for a variety of dominantly inherited, or gain-of-function and potentially recessively inherited loss of function liver and metabolic disorders. Will LNP delivery of gene editing payloads advance and provide enhanced therapeutic benefit beyond oligonucleotide (ASO, siRNA) and adeno-associated virus (AAV) delivered viral gene therapy and address the significant remaining unmet needs in a wide array of patients afflicted with hepatology and metabolic disorders? As Mia Hamm reportedly said (and certainly demonstrated), “success breeds success.”
“The CRISPR-Cas9 approach used for NTLA-2001 is modular and has the capacity to be adapted to treat a wide range of diseases with simple replacement of the sgRNA. …. these programs may make use of the potential not only to knock out expression of harmful protein products but also to insert genes to produce functional proteins where mutations cause pathologic deficiencies.”
Gillmore JD, Gane E, Taubel J, et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N Engl J Med. 2021;385(6):493-502. doi:10.1056/NEJMoa2107454