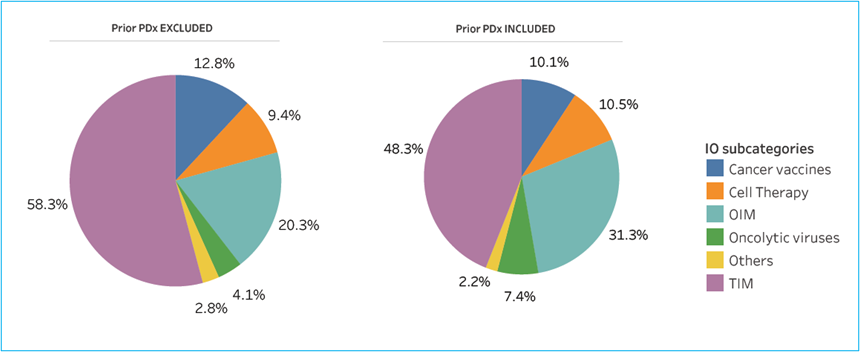
Immuno-Oncology (IO) is now a dominant force and major market in Oncology. And yet, notwithstanding the success of the immune checkpoint inhibitors (ICI), and despite significant efforts to find the next big thing in IO (billions of dollars every year over more than a decade, with tens of thousands of patients having participated in thousands of early and late-stage clinical trials), in the US we have had a limited number of additional approved IO products beyond a handful of cell therapies and several T-cell engagers, one next generation ICI (anti-LAG3), one oncolytic virus (Imlygic) and one cancer vaccine (Provenge). (See CRI data below.)
Despite multiple innate immunity agents demonstrating significant efficacy in preclinical trials, as with adaptive immunity agents the ability to translate success in animal models into meaningful clinical activity has been challenging. Hence, investor and deal-making activities have not fostered a strong environment for advancing drugs modulating innate immune pathways. In addition, failures of various key programs targeting cytokines, TLRs, STING, and of course IDO, to name but a few high-profile examples, have dampened the industry’s appetite. Finally, enthusiasm perhaps has been further tempered by studies that have reported that innate immune signaling may contribute to tumor promotion, resulting in added skepticism around the clinical significance and potential of this approach.
This session brings together a diverse array of biotech with a range of platforms and modalities to modulate innate pathways towards the goal of enhancing the ability of the patient’s immune system, with or even without checkpoint inhibitors, to control cancer.