Authored by Cello Health, now part of Lumanity
Dr. Adrian Bot (VP of Translational Medicine – Kite, a Gilead Company) provides an update on the factors determining durable responses achieved in relapsed/refractory (R/R) large B cell lymphoma patients treated with YESCARTA @ the CAR-TCR Summit Europe 2020.
Axicabtagene ciloleucel (Axi-cel), marketed as YESCARTA, is a first-in-class anti-CD19 CAR-T cell product, approved for treatment of R/R large B cell lymphoma (EU and US).

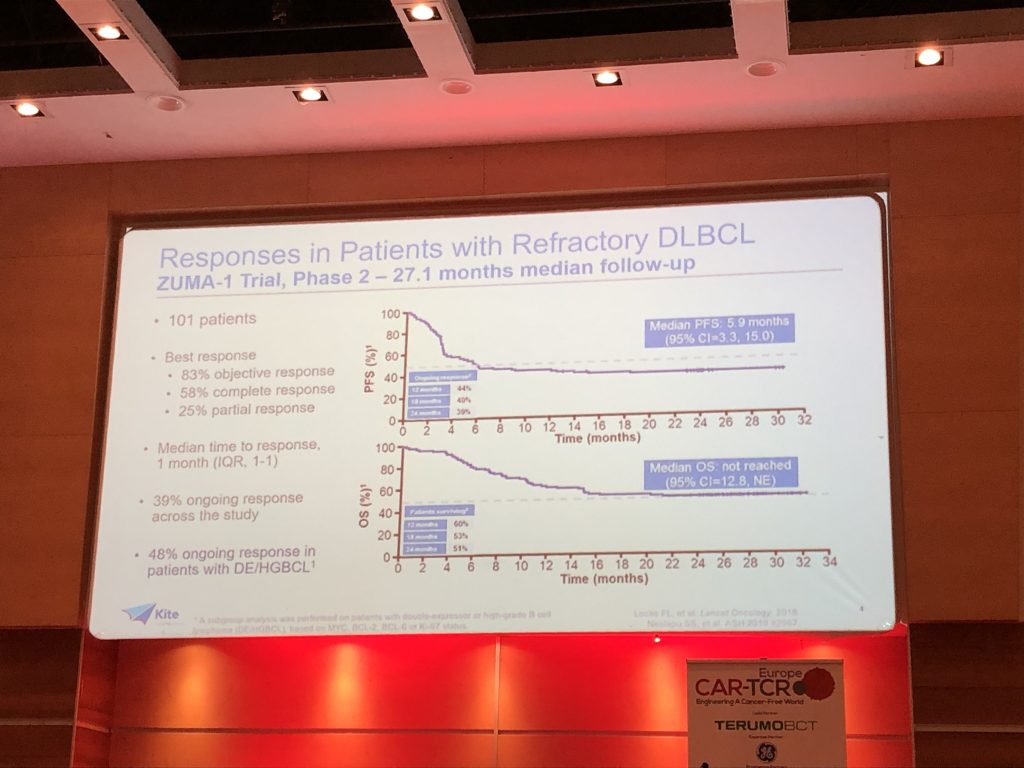
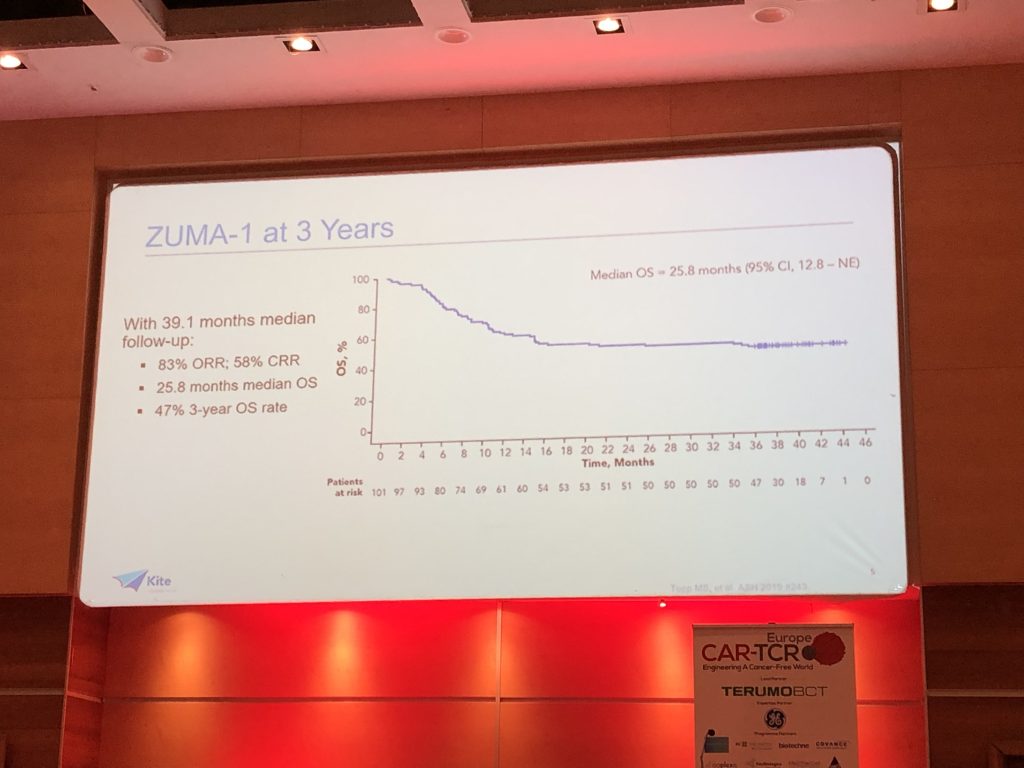
At a 27-month follow up in the ZUMA-1 multi-center registrational trial, 39% of the treated patients were still in response, indicating a stabilization of the Kaplan-Meier curve.
Key findings from a comprehensive retrospective multi-variate analysis of the ZUMA-1 trial demonstrate:
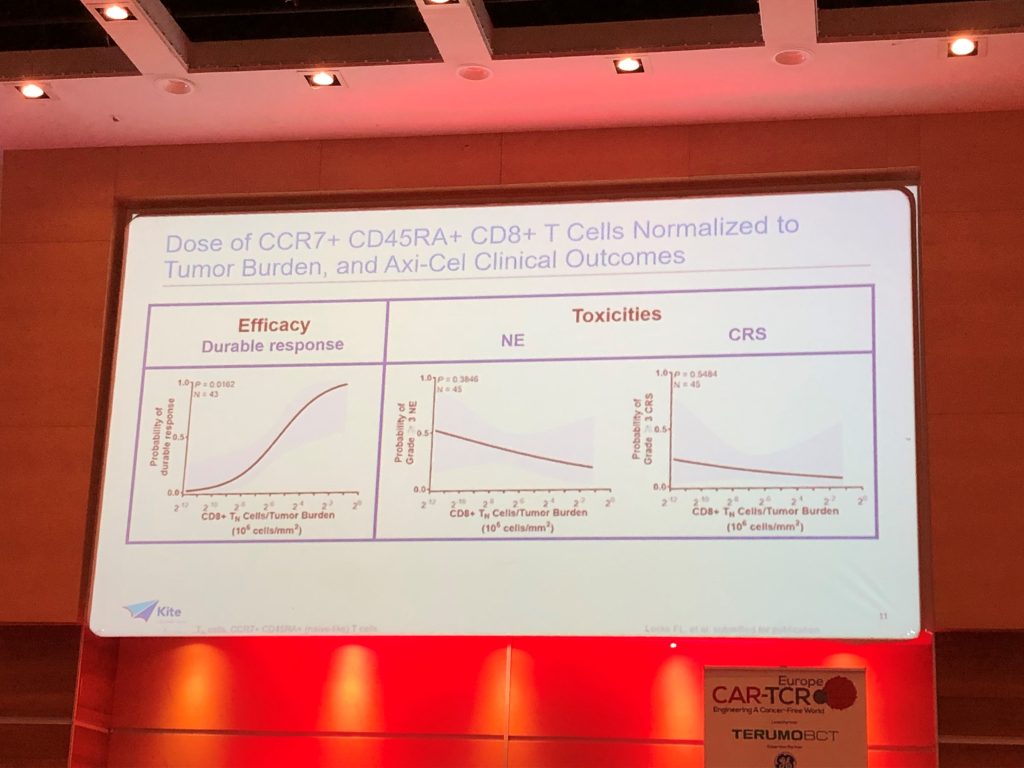
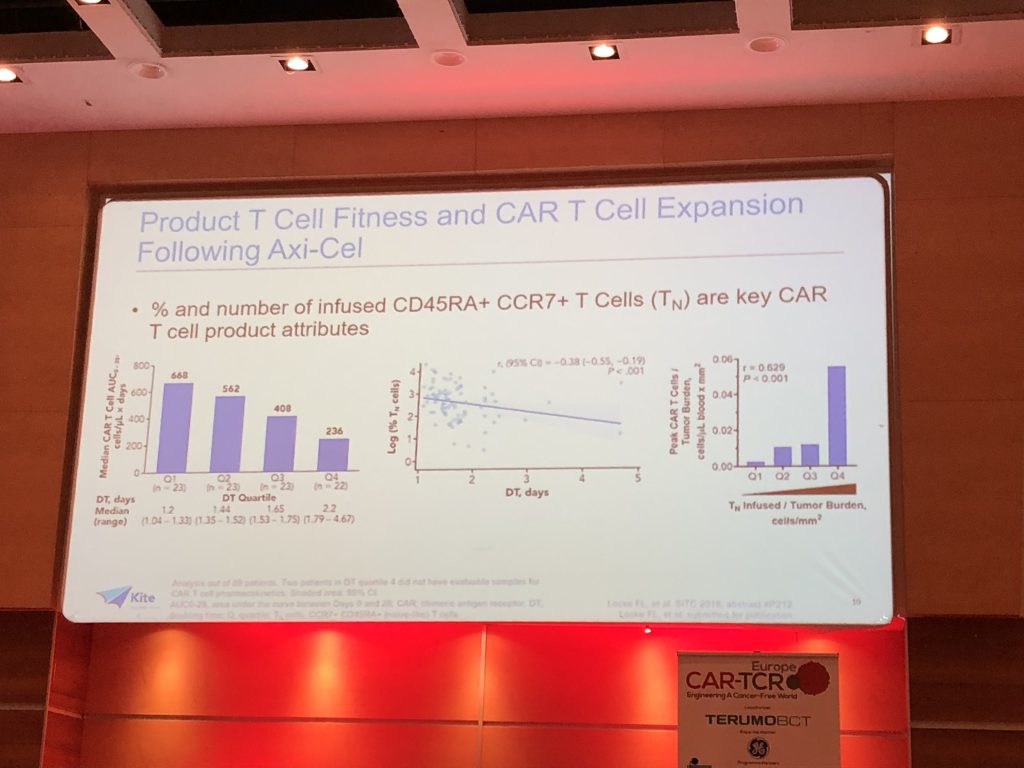
(1) The higher the level of the CCR7+ CD45RA+ CD8+ T cell population within the infusion product, the higher the probability of the patient achieving a durable response
(2) A median 39.1-month follow up of patients enrolled in the ZUMA-1 trial demonstrated a 25.8-month median OS in the YESCARTA treatment arm
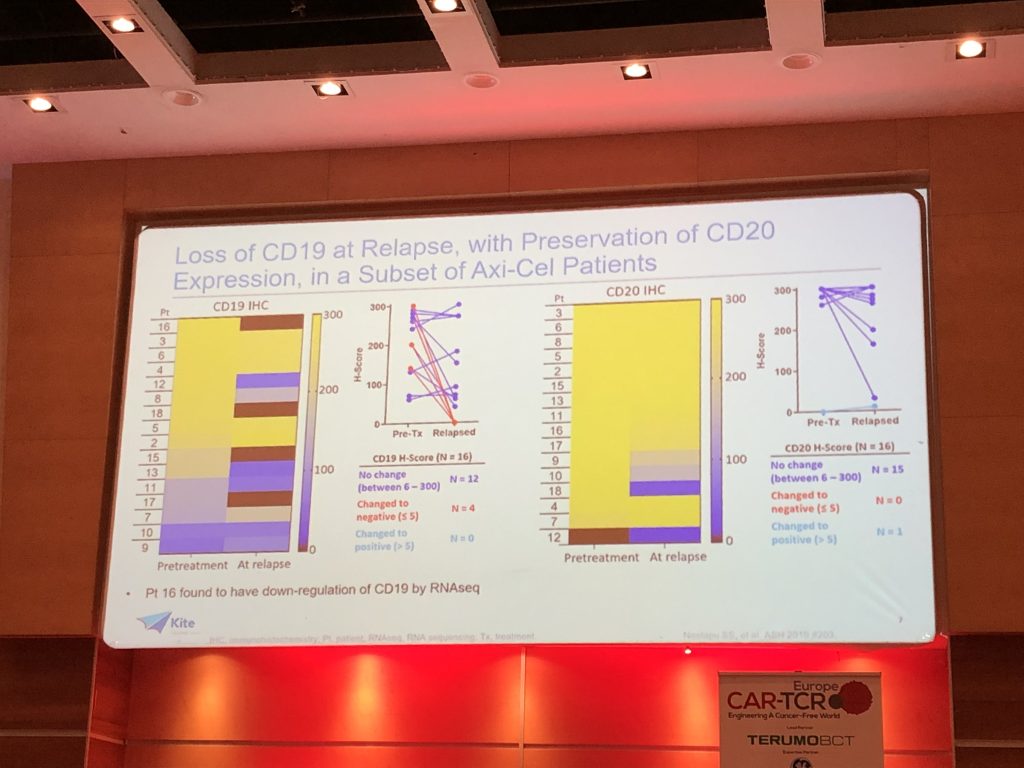
(3) Loss of CD19 from the B-cell surface and preservation of CD20 on the cell membrane was found in a subset of patients who relapsed whilst on YESCARTA. This may reveal a resistance mechanism that contributes to patients becoming refractory to CAR-T therapy
(4) Unsurprisingly, product T Cell fitness and number correlates with reduced tumour burden
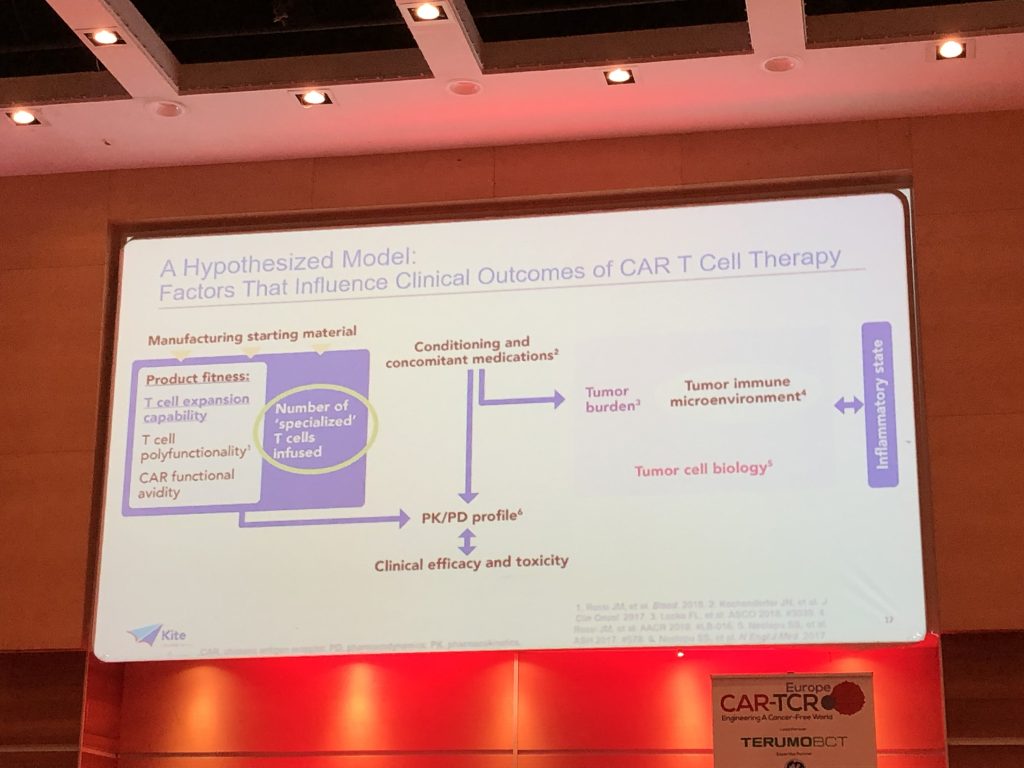
A hypothesised model detailing the factors that are proposed to influence clinical outcomes of patients receiving CAR-T therapy can be found below:
{ “We have assessed the major mechanisms that potentially contribute to resistance to CAR-T therapy and identified markers of durable responses”