Authored by BresMed, now part of Lumanity
COVID-19 has intensified many existing trends. One that is particularly relevant to this public health crisis is the need to accelerate the approval of innovative technologies. Even before the pandemic, regulatory initiatives by the FDA and the EMA sought to provide earlier conditional approval of innovative therapies for some rare, chronic and terminal conditions. Similarly, health systems have sought to increase access to new therapies via mechanisms such as the Temporary Use Authorizations in operation in France, the compassionate use programs (Härtefallprogramme) in Germany and the Cancer Drugs Fund in England.
The intense pressure exerted by COVID-19, when combined with the UK’s desire to remain an attractive launch market post-Brexit, have spurred further innovation and collaboration among decision makers. The lessons learned and applied under this pressure should enhance decision making in the UK – and perhaps these principles might be applied in other countries.
UK developments: applying lessons from the COVID-19 vaccine to other innovative products
COVID-19 and Brexit have forced the UK regulator (the MHRA), the English HTA body (NICE) and the English payer (the NHSE) to recognize and address limitations in the current approach to authorizing new and innovative therapies. In November 2020, this was defined as advanced therapy medicinal products [ATMPs], gene therapy products, vaccines, genomics, in vitro diagnostics and AI algorithms.1 The release of ILAP guidance in late December 2020 brought a much broader set of criterion (see below).2
The COVID-19 pandemic led to encouraging and enhanced communication between NICE, the NHSE and the MHRA, helping to develop the regulatory solutions needed to enable rapid access to therapies. In reviewing the overlaying technology development and regulatory pathways, the three organizations have jointly noted the need to:
- Support the innovation of products and new regulatory process tools and pathways
- Establish a more streamlined process
- Implement regulatory flexibilities and encourage new ways of working with other agencies
- More efficiently manage product lifecycle changes while also integrating good practice guidelines and licensing considerations
Greater flexibility in the regulatory process and NICE and the NHSE embracing a more adaptive approach to authorization and access fits with the need to innovate post-Brexit to ensure realism in NICE’s stated goal to: ‘encourage companies to launch their products first in the UK’.3
To meaningfully accelerate access, it will be necessary to not only enhance processes but also to improve collaboration – collaboration between manufacturers and the central decision makers (regulator, HTA body and payer), and, of course, with patients and healthcare professionals.
The new Innovative Licensing and Access Pathway proposed by NICE and the MHRA (figure below) seeks to achieve this greater flexibility and collaboration. More information is expected to become available on the planned use of the Innovative Medicines Fund to support this pathway during consultation later this quarter.
New Innovative Licensing and Access Pathway
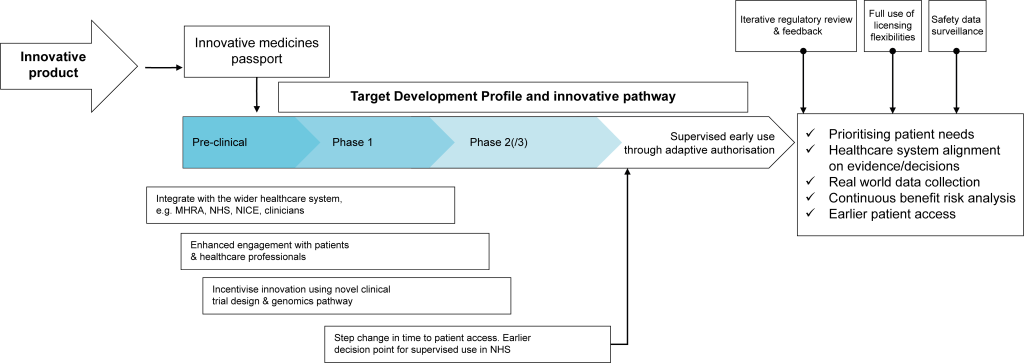
Source: NICE Webinar (2020)1
ILAP criterion for pathway use

What can manufacturers do to prepare?
All companies planning to bring an innovative product to market should be thinking through how to make the most of the opportunities early access routes afford, and how to best design their evidence generation strategy to enable access. This includes considering early access routes in advance, allowing at least 6 months from initiation of negotiations to patient supply.
Drivers that could motivate companies to explore opportunities for accelerated access include patient needs, the need to control development costs, and aggressive competitor launch timelines. The lessons learned through COVID-19 should be harnessed to allow accelerated access across markets. We suggest companies consider three actions:
- Keep up to speed with regulatory and reimbursement policy and process developments
- Shape the global policy context through proactive publication, stakeholder engagement and consultation response
- Leverage real world evidence and pragmatic clinical trial designs to capitalize on increased flexibility from decision makers and accelerate evidence generation
Now is a time of great change. In the UK, the Innovative Medicines Fund consultation is starting, the Innovative Licensing and Access Pathway2 is launching and the MHRA consultation4 on guidance for the use of registry-based studies in licensing has just closed. In Europe, the parallel EMA consultation on the use of registry-based studies5 will be producing guidance later in 2021, and the Spanish government has set up REvalMed to consolidate therapeutic positioning reports in the Spanish national health system (Sistema Nacional de Salud, SNS) as reference instruments for evaluating the cost effectiveness of medicines. In the US, ICER has launched a new analytics platform to accelerate real-world application of its findings.
It is an absolute must for manufacturers and their partners to keep up to speed with the many regulatory and reimbursement developments on the horizon, and to play an active role in consultation and the academic debate that will shape the landscape in years to come. On an individual asset level, the need to leverage real world evidence in both regulatory and reimbursement contexts has never been clearer. We recommend planning your launch activities at least a year before your regulatory submission and ensuring that anticipating the needs for later reimbursement play a central role in this planning.
Contact us for more information, or for help as you plan your evidence and launch strategy.
Abbreviations
AI, artificial intelligence; EMA, European Medicines Agency; FDA, Food and Drug Administration; HTA, health technology assessment; ICER, Institute for Clinical and Economic Review; MHRA, Medicines and Healthcare products Regulatory Agency; NHSE, National Health Service England; NICE, National Institute for Health and Care Excellence.
References
- National Institute for Health and Care Excellence (NICE). 2020. Webinar: NICE’s Life Sciences offer now and in the future: Learning from COVID-19. Available at: https://www.youtube.com/watch?v=nD_lZ75eUpE. Accessed: 25 January 2021.
- MHRA. 2020. Guidance: Innovative Licensing and Access Pathway (ILAP) for medicines. Available at: https://www.gov.uk/government/publications/innovative-licensing-and-access-pathway-ilap-for-medicines. Accessed: 25 January 2021.
- National Institute for Health and Care Excellence (NICE). 2020. Reviewing our methods for health technology evaluation: consultation. Available at: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/chte-methods-consultation. Accessed: 26 November 2020.
- MHRA. 2020. Draft guidance on randomised controlled trials generating real-world evidence to support regulatory decisions. Available at: https://www.gov.uk/government/consultations/mhra-draft-guidance-on-randomised-controlled-trials-generating-real-world-evidence-to-support-regulatory-decisions. Accessed: 26 November 2020.
- European Medicines Agency (EMA). 2020. Guideline on registry-based studies – launch of public consultation. Available at: https://www.ema.europa.eu/en/news/guideline-registry-based-studies-launch-public-consultation. Accessed: 26 November 2020.