Authored by Cello Health BioConsulting, now part of Lumanity
As we leap into the new decade, anyone who has a stake in oncology drug development has to be keeping an eye or two on the rapidly burgeoning cancer cell therapy space. I lead Cello Health BioConsulting’s cancer cell therapy practice, and rather than attempt to summarize all that’s happened since the groundbreaking work from investigators such as Zelig Eshhar, Steve Rosenberg, Carl June, and many others, I’ve decided to dive right in to where we are today and where things seem to be heading in this space with semi-regular (dare I say weekly?) posts that you can digest in five minutes or less. My assumption is that you, the reader, has a working knowledge of this market, both from the technical and commercial perspectives, and are tuning in to capture additional insight to help round out or challenge your thinking.
For my inaugural post, I’d like to muse on allogeneic CAR-T and cell therapy. In reading a recent Nature Review (subscription required) on the subject published by Stephen Grupp and others, I was struck by how fundamentally different allo and autologous cell therapy is from a biological perspective, and the positioning and partnering implications of these differences. Rather than get into the technical aspects of this (which are covered nicely in the review article), I think its important simply to point out that, whereas auto therapy can and should persist in the patient for a long time, in-kind allo assets likely will not due to the perhaps insurmountable challenge of rendering it invisible to the host immune system and the host immune system invisible to it. Regardless of whether this turns out to be true or not in the long run (indeed, many academic investigators and commercial entities, private and public, are investing their resources to address this exact hurdle), the expectations that capital markets seem to have in terms of allogeneic CAR-Ts like those being developed by Cellectis or Precision as providing a similar level, not only of efficacy, but durability, to that of their auto counterparts is at best misplaced and at worst damaging to the innovation engine.
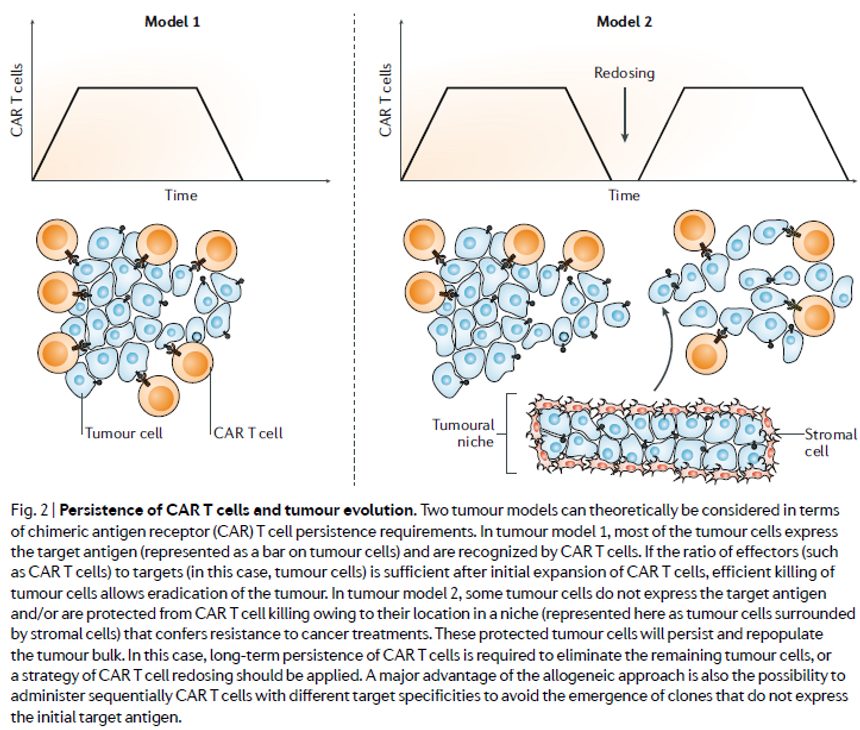
But do responses to allo CAR-T need to be as durable as those with auto products? Are the manufacturing advantages afforded by donor-derived, or perhaps even truly ‘off-the-shelf’ iPSC-derived, therapies relative to patient-derived counterparts in terms of reduced complexity, biologics-like COGs, and product homogeneity sufficient to facilitate re-dosing (assuming, as the review article points out, donor- specific anti-HLA antibodies (DSAs) don’t get in the way of doing so) such that an allo approach need not provide T-memory cell-mediated durability in order to achieve clinical utility. Indeed, a cell therapy amenable to re-dosing wouldn’t require the entire tumor and its associated mets to be snuffed out in one shot, but rather debulked and then perhaps struck again with a consolidation dose (see figure from the review below). Assuming this is the case, its also reasonable to assume that allo therapies would play a role that is complementary to rather than competitive with their auto counterparts.
There are many considerations that one can think of as critical determinants for whether or not allo CAR-T and other allo cell therapies can in fact play a role in the treatment algorithm (e.g. as maintenance, bridging, and/or outpatient treatment), even in the absence of high ORRs with demonstrated durability. For one thing, to what extent can allo therapy be administered in the absence of overtly toxic myelosuppressive conditioning therapy? This gets at the question of what conditioning therapy actually does (clear the lymphocyte ‘compartment’ for new T-cells, dampen the host immune response to facilitate engraftment, minimize risk of graft-mediated allo-reactivity, etc.). It also raises the question of whether it is myelosuppression or sequelae associated with the anticancer response itself (e.g. CRS) that puts patients in the ICU and pushes treatment costs beyond that allotted to US tertiary centers by the current outpatient-like DRGs (a major reason why hospitals are losing money on CAR-T at present). This then raises questions about which settings (e.g. post alloSCT, debulked solid tumors, maintenance therapy, etc.) or enabling modifications (e.g. TRAC, B2M, or CD52 KOs) must be leveraged in order to achieve clinical utility, let alone durability, with a non-self cell therapy product.
But let’s assume these issues are worked out. When (if?) the dust settles on what can rightly be call a renaissance in cancer cell therapy, one need not necessarily envision a world that is either auto or allo, but both. Especially if we were to hit the pause button on future breakthroughs akin to that achieved with the discovery of anti-CD19 and now anti-BCMA CARTs and instead must adjust our thinking to allow for cell therapy as one of many modalities from which patients and their providers have to select from.