Authored by BresMed, now part of Lumanity
Therapies designed to target cancers with specific molecular signatures have reshaped the landscape of oncological drug development.¹ The advent of tumour-agnostic therapies represents both a major opportunity for patients to receive therapies targeted to their specific disease on a molecular level and an important step towards discovering biomarkers of response.
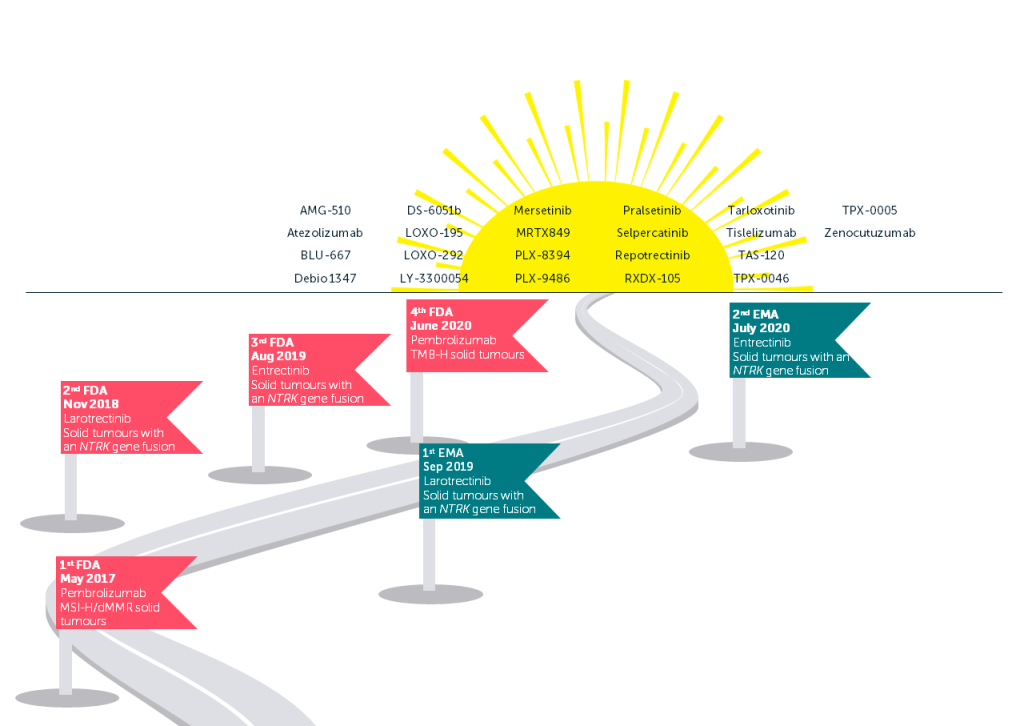
The tumour-agnostic approach establishes the effects of tumour type and context and makes clear the mechanisms of treatment resistance across a variety of cancer types. Advances in next-generation sequencing have led to an increase in the identification of molecular alterations across tumour types. This is now also improving access to the testing needed for patients to receive these therapies.² As the next generation of tumour-agnostic therapies make their way through clinical trials (see figure on page 2), we should be asking ourselves what we can do to prepare for the upcoming challenge of reimbursement in this space, and what lessons can be learnt from the experience of the tropomyosin receptor kinase (TRK) inhibitors larotrectinib and entrectinib.
Over the coming months, we will be releasing five short papers to provide some thoughts from our own experience in tumour-agnostic treatments. We will discuss the challenges that arise when developing evidence and strategies for the health technology assessment (HTA) of regimens in site-agnostic indications, outlining key points to consider when creating evidence dossiers and engaging with these processes. We will illustrate our recommendations for how to overcome common challenges by drawing on our own experience working with tumour-agnostic products and reviewing past HTAs.
Tumour-agnostic treatment landscape
While the challenges encountered when seeking reimbursement for tumour-agnostic therapies are similar to those for many treatments in the oncology space (particularly those targeting small patient populations), there are some additional challenges that link to the need for mass genomic testing associated with these therapies and the trial design predominantly used to produce evidence in this space. Early planning of your evidence generation, economic modelling, clinical and payer engagement, and HTA strategy will be even more crucial to achieving reimbursement than usual.
The majority of tumour-agnostic therapies are tested using basket trials, which include patients with multiple tumour types all with the same biomarker relevant to the target pathway that the proposed therapy is designed to inhibit. These trials use a centralized screening platform to identify eligible patients and a common protocol for different sub-studies, which may each focus on patients with specific markers or tumour types.5 Most basket trials are exploratory (Phase I or II), open-label, use frequentist decision rules and lack a control group.6 Although it is not impossible to conduct basket trials with a control arm, the vast majority of tumour-agnostic trials have not been designed this way; this is due to difficulties in defining the comparator arm across tumours, the rarity of the relevant biomarker in some tumour types, and the ethical considerations given the size of benefit expected for the therapies licensed so far.
What is a basket trial?
In basket trials, patients all receive the same treatment that targets the specific mutation or biomarker found in their cancer.⁷
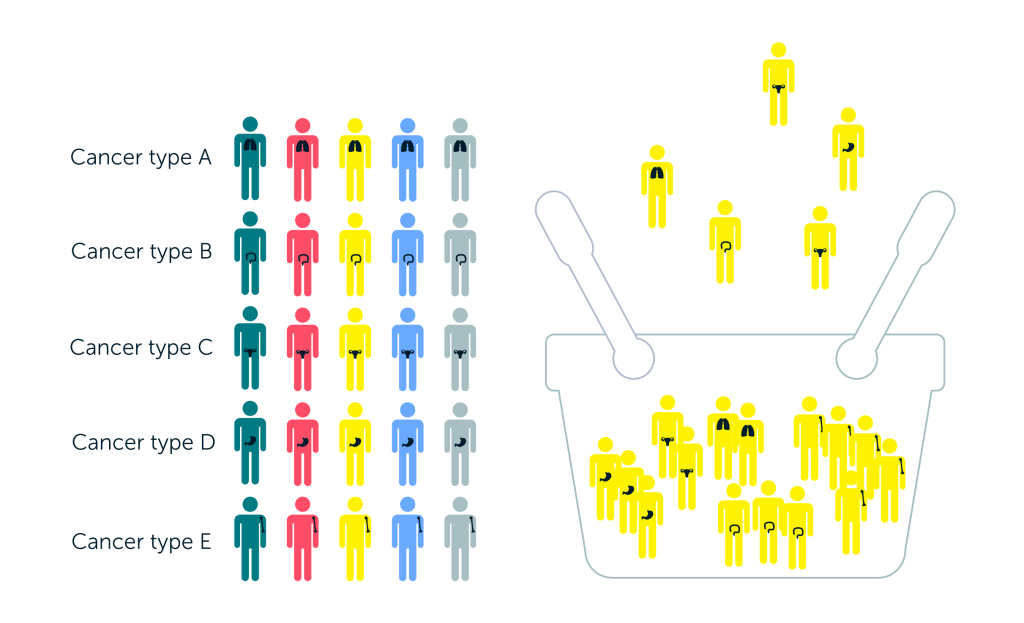
Single protocol = simplified process
More patients eligible to enter
Adaptive – patient numbers can be increased for tumours with promising results and reduced for those with low efficacy
Potential to include Bayesian or frequentist tests for heterogeneity in outcomes within trial design
Disadvantages
Assumes that the biomarker is a good predictor of response
May not recruit a sufficient number of patients for all tumour types or mutations of interest
Differences in standard of care across tumour types limit the possibilities to include a common comparator arm
Source: Murphy et al., 2020.8
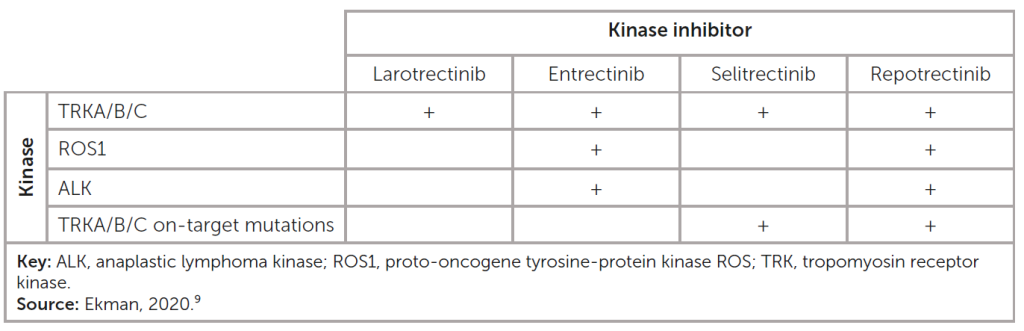
Of particular importance for the applicability (and development) of tumour-agnostic therapeutics is the variability of response across different indications (see table), given the unique signalling context not only for a specific cancer but also for an individual patient. In addition, these agents have varying target specificities, which affect their efficacy.
Kinase activity of TRK inhibitors in clinical use and under development
Key challenges associated with tumour-agnostic treatments
Across five key factors, the HTA process for tumour-agnostic therapies is more complex than that for standard oncology treatments and for rare diseases.
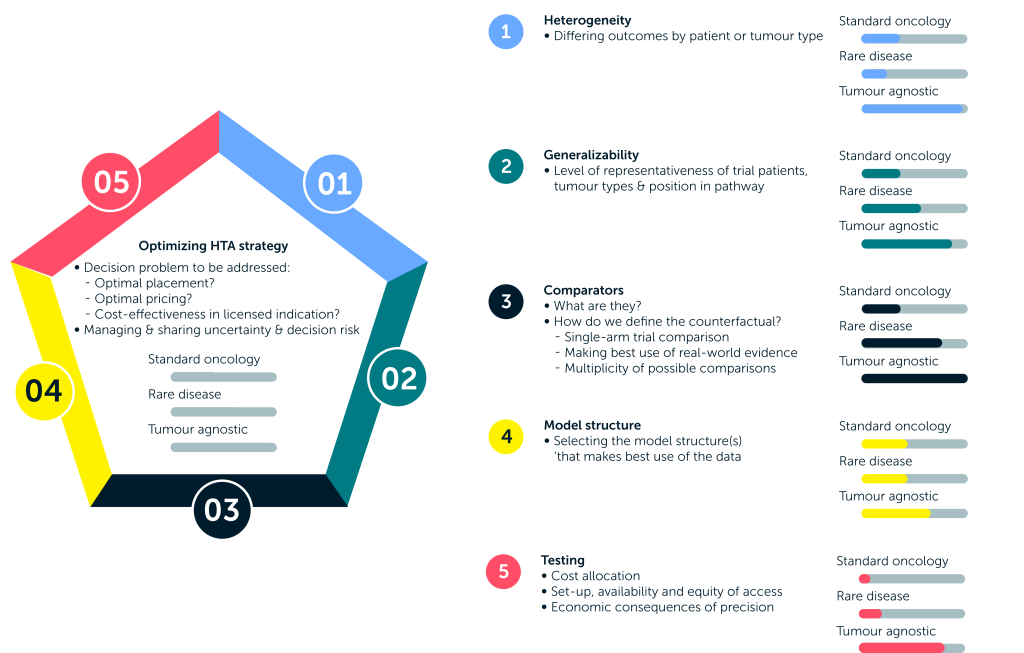
Given this wide array of challenges, how can we assess the effectiveness of these innovative and potentially life-saving treatments in tumour-agnostic indications in a valid and reliable manner? In the next paper, we dive into the core issues surrounding this challenging task – namely, the difficulties in considering the inevitable heterogeneity of patient populations and determining how generalizable the results of such clinical trials are to those expected to be treated in practice – and outline the measures you can take to handle the uncertainty generated in your reimbursement submissions.
For information on how we can support your next tumour-agnostic therapy, please contact us.
What are tumour-agnostic therapies?
Tumour-agnostic therapies target specific genomic anomalies or molecular features regardless of tumour site of origin.3, 4 The rationale for this approach is as follows:
- Tumour cells contain one or more genetic alterations, often causing certain gene products to be overexpressed
- The same few alterations are commonly found across a variety of cancer types
- The presence of such alterations is likely to predict response to a therapy
References
- Offin M, Liu D and Drilon A. Tumor-Agnostic Drug Development. American Society of Clinical Oncology Educational Book. 2018; (38):184-7.
- Lavacchi D, Roviello G and D’Angelo A. Tumor-Agnostic Treatment for Cancer: When How is Better than Where. Clin Drug Investig. 2020; 40(6):519-27.
- OncologyPRO. Tumour-Agnostic Treatment. Available at: https://oncologypro.esmo.org/oncology-in-practice/anti-cancer-agents-and-biological-therapy/targeting-ntrk-gene-fusions/overview-of-cancers-with-ntrk-gene-fusion/precision-medicine/tumour-agnostic-treatment. Accessed: 7 July 2021.
- Looney A, Nawaz K and Webster R. Tumour-agnostic therapies. 2020. Available at: https://www.nature.com/articles/d41573-020-00015-1. Accessed: 29 July 2021.
- Blagden SP, Billingham L, Brown LC, et al. Effective delivery of Complex Innovative Design (CID) cancer trials-A consensus statement. Br J Cancer. 2020; 122(4):473-82.
- Meyer EL, Mesenbrink P, Dunger-Baldauf C, et al. The Evolution of Master Protocol Clinical Trial Designs: A Systematic Literature Review. Clinical Therapeutics. 2020; 42(7):1330-60.
- NIH National Cancer Institute. Basket trial. Available at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/basket-trial. Accessed: 29 July 2021.
- Murphy P, Glynn D, Dias S, et al. Modelling approaches for histology-independent cancer drugs to inform NICE appraisals. 2020. (Updated: 28 February 2020) Available at: https://www.nice.org.uk/Media/Default/About/what-we-do/Research-and-development/histology-independent-HTA-report-1.docx. Accessed: 7 July 2021.
- Ekman S. How selecting best therapy for metastatic NTRK fusion-positive non-small cell lung cancer? Translational lung cancer research. 2020; 9(6):2535-44.