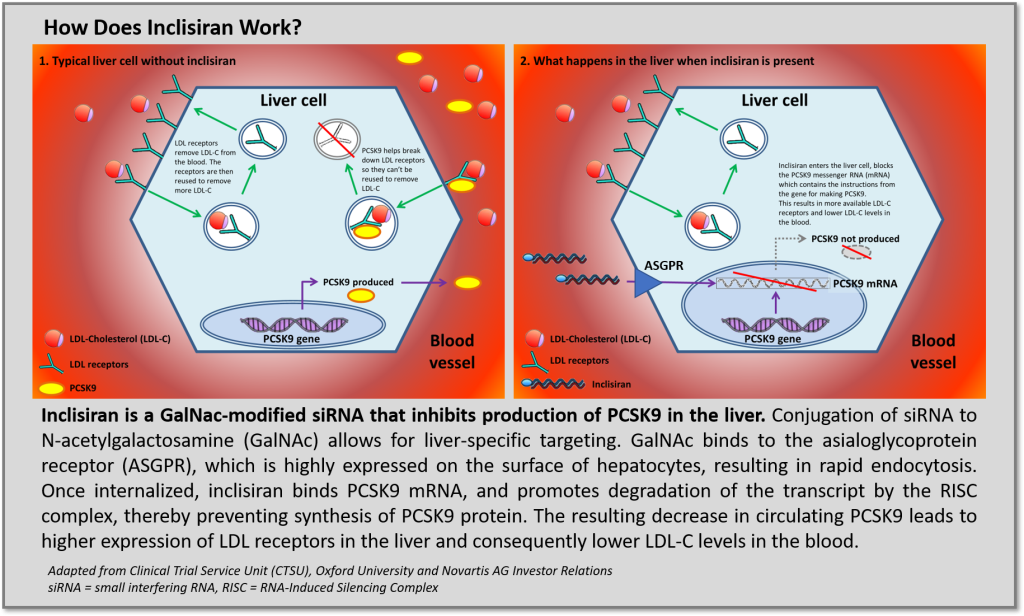
Authored by Cello Health BioConsulting, now part of Lumanity
In the last several years, RNA-targeted platforms yielded FDA/EMA approved therapies for ultra-rare metabolic and neurodegenerative diseases that address significant unmet medical needs justifying high therapeutic value ($400 to >$800k annual cost of therapy). All the approved approaches utilize synthetic oligonucleotides but employ different chemistries, delivery vehicles and therapeutic mechanisms: antisense oligonucleotides (ASOs) target messenger RNA (mRNA) for RNAse H dependent degradation or can be designed to upregulate expression though exon skipping or splice modification. Another more nascent approach, small interfering RNA (siRNA) technologies, degrades mRNA transcripts by activation of the recyclable RNA-Induced Silencing Complex (RISC) and has demonstrated competitively favorable tolerability and pharmacokinetic profiles facilitating infrequent, potentially “vaccine-like” dosing intervals due to recycling of the RISC complex. Although efforts to develop oligonucleotide-based therapies for indications beyond the liver have largely been stymied (aside from targets amenable to local delivery), improvements in chemistry and stability are further validating and providing great enthusiasm for these therapeutic platforms to extend clinical benefit beyond ultra-rare diseases and address unmet needs for more common medical disorders prevalent in broader (i.e., large) patient populations. This may soon be a reality with the imminent approval of Novartis acquired, siRNA PCSK9 inhibitor inclisiran, developed by The Medicines Company and Alnylam.
Recently published results in NEJM (refs 1, 2) support enthusiasm for the FDA to approve inclisiran for the treatment of hypercholesterolemia and potentially secondary cardioprevention. As a new therapeutic modality, first generation siRNA drugs faced safety concerns (ref 3), however inclisiran utilizes new oligonucleotide chemistries to improve both adverse event (AE) and efficacy profiles. Specifically, GalNAc conjugation targets delivery to asialoglycoprotein receptors (ASGPR) on hepatocytes to significantly improve the therapeutic index of inclisiran. Across the series of ORION trials, the rate of treatment-emergent AEs and serious adverse events occurred with similar frequency between inclisiran treatment arms and comparative placebo treatment arms. No signals of inclisiran causing liver effects, renal or muscle injury, thrombocytopenia, or malignancy were observed. Furthermore, although rates of injection-site reactions were higher among inclisiran treated groups, these were limited to mild/moderate cases with no severe or persistent injection-site reactions being reported.
Regarding efficacy, pivotal phase 3 data demonstrate ~50% reduction in LDL-C on top of conventional lipid reducing therapy (i.e., maximal tolerated statins) in treatment groups across multiple patient populations (Figure 1) including patients with: heterozygous familial hypercholesterolemia (HeFH, ORION-9) (ref 2), atherosclerotic cardiovascular disease (ASCVD, LDL-C ≥70 mg/dL, ORION-10) (ref 1), and ASCVD Risk Equivalent (T2DM, FH, or a 10-year risk of a cardiovascular event of ≥20% as assessed by the Framingham Risk Score for Cardiovascular Disease or equivalent, LDL-C ≥100mg/dL, ORION-11) (ref 1).

Figure 1. Change in LDL-C (left) and Absolute LDL-C (right) Level in ORION-9 (HeFF), OPRION-10 (ASCVD), and ORION-11 (ASCVD Risk Equivalent) Phase 3 Trials of Inclisiran. Adapted from sources 1, 2
However, with efficacy and safety roughly equivalent to already approved therapies in terms of % LDL-C lowering (Amgen’s Repatha and Sanofi/Regeneron’s Praluent), Novartis has a challenge in front of itself in order to exploit their $9.7B investment. Novartis is attempting to enter a broad and incredibly challenging space and must execute a thoughtful go-to-market strategy in order to ensure a successful launch that captures market share from competitors who themselves are struggling to gain traction. Inclisiran’s differentiating qualities and lessons learned from less than stellar launches of the mABs may just be the perfect storm for Novartis to launch a winner.
Compared to bimonthly or monthly dosing of Repatha and Praluent, inclisiran’s twice annual “vaccine-like” dosing schedule may be enough differentiation for payers to strongly consider making inclisiran a preferred therapy in the hypercholesterolemia space. Where the existing mABs are self-administered, recent analysis (ref 4) suggests Novartis will push for a physician-administered ‘buy and bill’ model, which they argue will improve compliance, and thus real-world outcomes, compared to self-administered regimens. To help prove this point, Novartis has teamed up with the UK’s National Health Service (NHS) to conduct a large-scale trial where up to an estimated 40,000 NHS patients across a variety of hypercholesterolemia settings may be administered inclisiran (ref 5). The collaboration encompasses three proposals: (1) a primary prevention clinical trial, (2) a population health access model for high-risk ASCVD patients (upon marketing authorization and NICE approval), and (3) a proposal to look at manufacturing synergies that could improve oligonucleotide manufacturing scale and efficiency. It remains unclear whether US payers will share the optimism that the NHS has towards inclisiran’s potential to make significant contributions to population health. However, this set of studies should provide a framework to collect evidence of cost effectiveness in terms of how improved compliance will translate into improved patient outcomes. Given how highly managed the cholesterol market is, especially in the US, payers will surely take note. Novartis is expected to launch inclisiran at a price below the ICER recommended price for PCSK9 antibodies and provide a unique contractual guarantee between manufacturer, physicians and payers to ensure compliance and offset costs on CV events. However, with ongoing outcomes trial not set to readout until 2024, we may have to wait for full MACE data before truly knowing how disruptive this new therapy and healthcare delivery model will be.
Oligonucleotide-based therapeutic programs continue to attract significant investments from large biopharma companies. The approval and successful launch of inclisiran will undoubtedly inflect even more value to the entire sector by serving as a bellwether that siRNA inhibition of liver targets is a derisked therapeutic platform. Beyond PCSK9, industry alliances and collaborations are furthering development of siRNA therapies against liver metabolic targets such as ApoB100-LDL, APOC3, ANGPTL3, Lp(a) and other metabolic mediators, all of which have blockbuster potential. At Lumanity, we are closely monitoring developments related to oligonucleotide-based therapies and will continue to publish short analyses on different segments within the space. Follow Lumanity and stay tuned as we release additional content relevant to exciting advances across technology platforms and therapeutic areas. If you have any questions or comments on this piece or want to learn how we might work together to advance your own therapeutic programs, please feel free to reach out.
1. N Engl J Med. 2020 Mar 18, PMID: 32187462
5. Novartis Press Release: January 13, 2020{
“After a groundswell of hype and a skeptical backlash, the pharmaceutical industry is learning how to leverage RNA interference in the clinic.”