Authored by Cello Health, now part of Lumanity
Really insightful talk delivered by Dr. Mythill Koneru (Chief Medical Officer – Marker Therapeutics) @ the CAR-TCR Summit Europe 2020 highlighting the advantages of Marker Therapeutics Multi-Tumour-Associated Antigen-Specific (MultiTAA) T Cells in multiple oncology-focused indications.
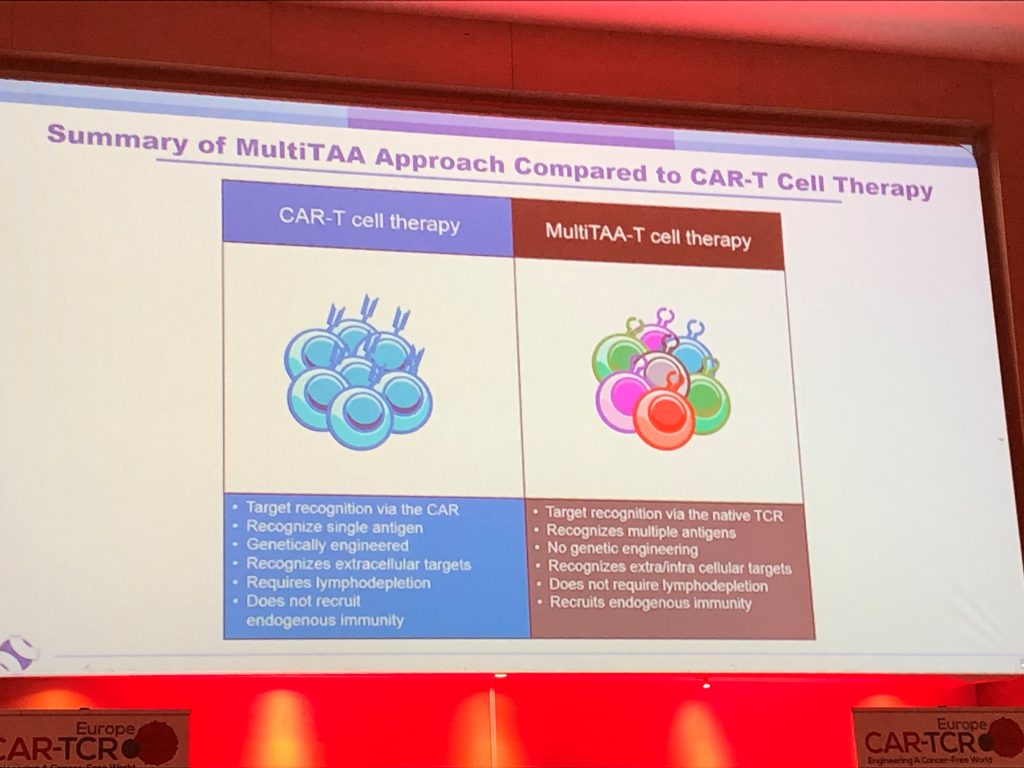
Tumours are heterogeneous in nature, commonly being comprised of many different cells expressing a multitude of different receptors on their surface. Unlike conventional CAR-T’s, which are genetically engineered to recognise a single receptor, Marker Therapeutics MultiTAA cell therapy is able to target multiple receptors simultaneously. This may drive a more durable response and result in fewer patients becoming relapse and refractory to treatment.
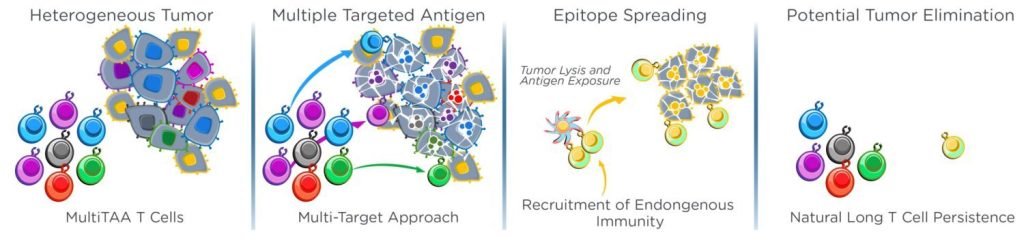
MultiTAA technology selects for and expands patient tumour-specific T cells by directly presenting a proprietary blend of tumour-associated antigens and cytokines in vitro, by-passing the need for expensive, time-consuming, and complex genetic modifications. This approach offers numerous advantages, including:
- A safer approach vs. CAR-T’s due to there not being a requirement for genetic modification / lymphodepletion
- A cheaper approach to CAR-T’s due to reduced manufacturing complexity
- An efficient route of administration, with the therapy being administered and available in an outpatient setting
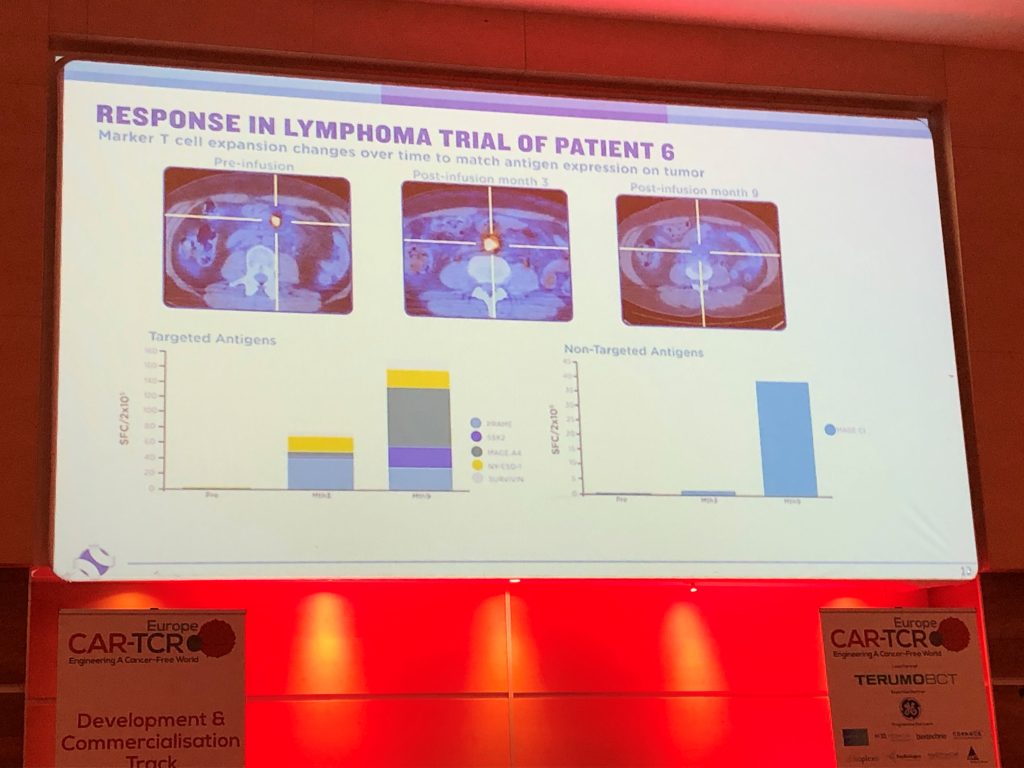
- MultiTAA’s offer a durable response, with lymphoma trials showing that patients receiving MultiTAA’s are relapse-free beyond 2 years
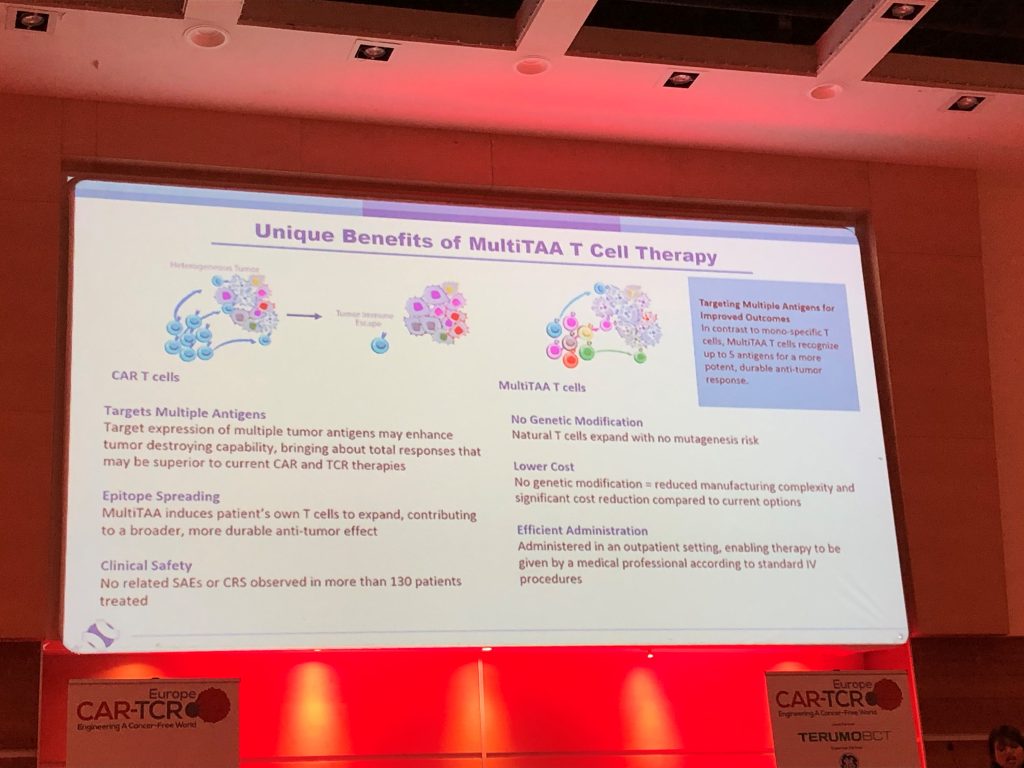
Additionally, MultiTAA clinical trials have shown evidence of ‘epitope-spreading’, a phenomenon that means that in addition to the tumour antigen-specific T cells contained in Marker Therapeutics infused cells, patients’ own T cells go on to recognise additional tumour-associated antigens and expand and contribute to a durable anti-tumour response.
The MultiTAA novel therapeutic approach is currently being investigated in numerous clinical trials to combat various cancer types.
{“Clinical trials have shown that around 30-50% of patients with lymphoma receiving CAR-T therapy relapse, with the majority relapsing within 1 year. In clinical studies, MultiTAA’s have shown promising response rates in lymphoma, with around 50-60% of patients showing complete response rates, which are highly durable, with some patients continuing to be relapse-free beyond 2 years”