The Spanish Ministry of Health has finally proposed a new governance structure to oversee health technology assessment (HTA) in Spain. We see a strong influence of the European Regulation on HTA: a statement is made on the identification of nine domains aligned with the EUnetHTA domains, and timelines will also be tremendously accelerated when a joint clinical assessment (JCA) is available. As we expected, the proposed framework is also similar to the structure of the National Institute for Health and Care Excellence (NICE) in the UK. Below, we share our thoughts on the draft Royal Decree.
Spain is in a public consultation period until 20 September following the publication in August of a draft Royal Decree on HTA. Stakeholders, organizations, and the Spanish public have until the September deadline to give feedback on the draft HTA proposal. The draft Royal Decree was issued by the Spanish Ministry of Health and unveils a new governance structure dubbed “HispaNice” due to its copycat features when compared with the National Institute for Health and Care Excellence (NICE). The proposals look set to govern future HTA decisions across Spain.1
Framework
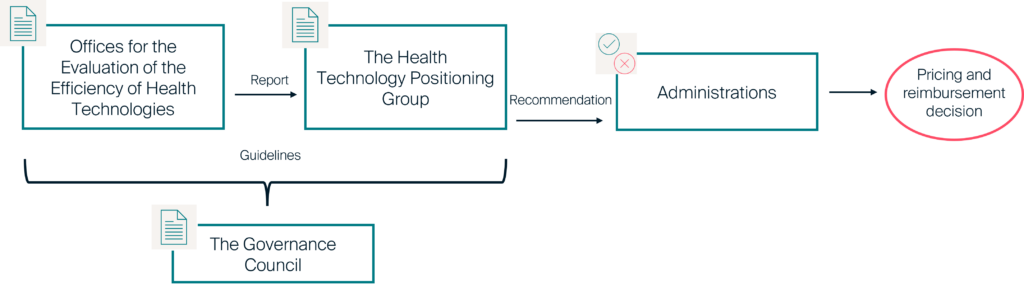
The draft Royal Decree provides a formal framework for the HTA process in Spain. The Spanish Agency of Medicines and Medical Devices (AEMPS, Agencia Española de Medicamentos y Productos Sanitarios), the national counterpart of the European Medicines Agency (EMA), will assume the new HTA responsibilities following the consultation. The proposed framework for the HTA process includes a governance body (“Governance Council“), two independent assessments – one for medicines and other health technologies (“Offices for the Evaluation of the Efficiency of Health Technologies”) and a positioning body (“Health Technology Positioning Group”). Here are the highlights.
Contact us
If you would like advice or help on navigating the EU HTA Regulation, please contact us.

The Governance Council
The Governance Council ensures the alignment of the HTA framework with the pharmaceutical policies of the Ministry of Health. The Governance Council is independent and all its members will not participate directly or indirectly in HTAs. The Council’s main functions will include adopting internal operating rules and defining the annual work programs. Additionally, at the request of the Offices and the Positioning Group, it will provide methodologic guidelines. The Governance Council may receive advice from the Advisory Committee for the Financing of Pharmaceutical Services (CAPF, Comité Asesor de la Prestación Farmacéutica del SNS) of the Spanish National Health System (SNS, Sistema Nacional de Salud), which currently provides support to the Interministerial Drug and Medical Devices Pricing Committee ( CIMP, Comisión Interministerial de Precios de Medicamentos y Productos Sanitarios).

Offices for the Evaluation of the Efficiency of Health Technologies
Two offices will conduct the HTA assessments, one office will do so for medicines and the other for health technologies. The creation of a specific office for health technologies represents a clear increase in the scope of assessments as only medicinal products are considered in the current evaluations. The two offices will conduct the clinical and the economic assessments, with the economic assessment including a cost-effectiveness assessment and a budget impact analysis. Importantly, the two offices will not take part in decision-making. Their evaluations will not contain judgments on the positioning in the SNS or on decisions about price or inclusion in the common portfolio of services. Once the reports are finished, the Offices will submit them to the Health Technology Positioning Group.
Before the assessment, the relevant Office will inform the manufacturer about the scope of the assessment and request a dossier containing complete and up-to-date information, data, analyses and other evidence. The Offices will complete their assessment reports, clinical and non-clinical, for drugs and health technologies in 90 calendar days after the decision has been communicated by the body responsible for authorizing them. In any case, where a joint clinical assessment is available, no more than 20 calendar days should elapse after the report has been published by the European Commission. These timelines can be extended by 30 days if additional information or analyses are required. The manufacturer will have 10 days to provide the additional information requested.
The assessment offices will ensure that patients, clinical experts and other relevant experts will have the opportunity to provide feedback on draft reports. Manufacturers will also have the opportunity to flag any technical or factual inaccuracies, as well as any information they consider confidential. However, manufacturers will not be able to comment on the results of the assessment. Once finished, the assessment reports will be published.
The draft Royal Decree allows for the potential use of real-world evidence (RWE) data. However, just how the assessments will incorporate the data has not been clarified. The Spanish Guidelines on Economic Evaluation for Drugs, published in March 2024, only considered the RWE data for external validation. Therefore, future guidelines should address the possible use of RWE data.2
The Offices may carry out advisory consultations at the request of the manufacturer. These consultations provide an early dialogue aimed at guiding future evaluations, which can cover any aspect of the HTA. Additionally, the offices will engage in the early detection activities of emerging technologies, propose methodological guidelines for technology assessment and cooperate with relevant bodies at the EU level to exchange scientific information in accordance with the terms set out in Regulation (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021.3

Health Technology Positioning Group
The aim of the Health Technology Positioning Group is to make a final recommendation based on the evaluation reports prepared by the Offices and taking into account additional information. The list of group members will be public and will consist of:
- A president
- A secretariat
- A representative of the General Sub-directorate of Pharmacy of the Ministry of Health
- Two representatives appointed at the proposal of the CIMP
- Seventeen representations of the autonomous regions (one representative for each autonomous region) appointed at the proposal of their respective Health Departments
- Two representatives of health professionals
- One specialist in health economics
- One representative of patient organizations
- One representative of consumer organizations
The last five roles will be appointed by the General Directorate of the Common Portfolio of Services of the National Health and Pharmacy System.
For specific aspects, the group may include ad hoc participation by experts, professionals and/or patients, who can verbally participate in the discussions while having no right to vote. Additionally, manufacturers may have an audience with the Health Technology Positioning Group to facilitate discussion with all stakeholders.
The Health Technology Positioning Group will reach its decision by consensus. If consensus is not possible, the decision will be based on a simple majority of the members. The recommendations of the Health Technology Positioning Group will inform the decisions of the Administrations, but will not constitute the decision itself. The Health Technology Positioning Group will provide justification for its recommendations based on the reports from the Offices. However, the Administrations are free to decide based on their own criteria. Finally, the recommendations of the Health Technology Positioning Group will be published by the entity responsible for decision-making on financing or incorporation into the portfolio as part of a reimbursement decision.
Figure 1: Health Technology Positioning Group decision process
Similarities with NICE
The proposed framework as expected, is similar to the structure of NICE in the UK. Previous communications declared intentions to create an HTA agency similar to NICE, plans that in recent years have been called “HispaNice.”4,5 The Spanish framework consists of a Positioning Group similar to the NICE committees, and the evaluation Offices are equivalent to the NICE External Assessment Group (EAG). Both the evaluation Offices and the EAG prepare a technical report without recommendations for the NICE committees/Positioning Group, which will provide a recommendation based on the assessment. However, neither recommendation is binding, and the reimbursement bodies can make their own independent decisions. Other similarities include a formal early dialogue between the manufacturer and the evaluation Offices.
The Positioning Group includes 25 members, similar to the number of members in the four NICE committees (24–26 members).6 Only one member of the Positioning Group committee is a designated health economist, although more could be expected if they act as representatives of the autonomous regions or of the Ministry of Health. Any economic assessment, and therefore, pricing and reimbursement decision, will require an understanding of the applied statistical approaches, their modeling and the uncertainty caused by the applied methods. Therefore, it is essential that the composition of the group reflects the required knowledge and capabilities, and that it consistently trains its members to understand the current methods of health technology economic evaluations.
Challenges
The proposed structure will replace the current Drug Evaluation Network (REvalMed – Red de Evaluación de Medicamentos), which has been conducting the positioning assessment reports (IPT – Informes de Posicionamiento Terapeutico) since 2020. According to the IPTs plan, the positioning assessment should be conducted in 90 days after the positive marketing decision from the Committee for Medicinal Products for Human Use (CHMP).7 However, according to the last Patients W.A.I.T. Indicator 2023 Survey, published in June 2024 by the European Federation of Pharmaceutical Industries and Associations (EFPIA), the time from CHMP approval to reimbursement is 661 days (period 2019–2022).8 This does not mean that the positioning report takes approximately 2 years. The reports take much less time. However, given the capacity constraints, not all treatments can be assessed immediately after their approval, and REvalMed has to decide which treatments to prioritize. The new proposed framework aims to complete the clinical and economic report in 90 days, i.e. the same timelines that REvalMed considered and failed to achieve. Therefore, it is not clear how the new framework will address the delays in drug access as no additional context is provided for the working framework of the Offices.
Regarding the structure of the evaluation Offices, the draft Royal Decree does not provide additional context for the expected design. An initial guess is that the evaluation Offices will follow a structure similar to the evaluation Nodes from REvalMed. The Nodes include 18 specialities and 143 experts – with roughly 15 experts per Node. Previous reports have shown concerns about the selection criteria of the members, given the apparent discretion of the administrations to incorporate experts. Most of the members from the evaluation Nodes belong to Hospital Pharmacy (around 66%), with only three members from Clinical Pharmacology and two health economists.9 The apparent professional bias and lack of transparency in the selection of its experts remain unsolved in the draft Royal Decree as it has not clarified the exact structure and framework of the Offices.9 However, even though the structure is not defined, the draft Royal Decree provides the Offices with the legal basis to accurately perform the HTAs, which its predecessor, REvalMed, lacked.
Despite the previous limitations, the new draft Royal Decree is moving in the right direction by overcoming REvalMed’s lack of activity and competencies regulation and legal basis, and the overall scattered and complicated structure of the HTA process in Spain.
References
- Ministerio de Sanidad “Proyecto de Real Decreto por el que se regula la evaluación de tecnologías sanitarias“, 12 August 2024, Available at: https://www.sanidad.gob.es/normativa/audiencia/docs/DG_54_24_Solicitud_informacion_publica_RD_EVALUACION_TECNOLOGIAS_SANITARIAS.pdf. Accessed: September 2, 2024.
- Ministerio de Sanidad “Guía de Evaluación Económica de Medicamentos,” 4 March 2024. Available at: https://www.sanidad.gob.es/areas/farmacia/comitesAdscritos/prestacionFarmaceutica/docs/20240227_CAPF_Guia_EE_definitiva.pdf. Accessed: September 2, 2024.
- European Commission “Regulation on Health Technology Assessment” 15 December 2021. Available at: https://health.ec.europa.eu/health-technology-assessment/regulation-health-technology-assessment_en. Accessed: September 2, 2024.
- Perez A. (2023). “El HispaNICE se vislumbra real en el SNS similar a la Airef y sistemático,” Redacción Médica, 19 July 2023. Available at: https://www.redaccionmedica.com/secciones/tecnologia/el-hispanice-se-vislumbra-real-en-el-sns-similar-a-la-airef-y-sistematico-1474. Accessed: September 2, 2024.
- Arganda C. (2023). “Funcas publica una propuesta para poner en marcha un ‘Hispanice’ a dos velocidades,” Diariofarma, 13 December 2023. Available at: https://diariofarma.com/2023/12/13/funcas-publica-una-propuesta-para-poner-en-marcha-un-hispanice-a-dos-velocidades. Accessed: September 2, 2024.
- National Institute for Health and Care Excellence (NICE), “Technology appraisal committee.” Available at: https://www.nice.org.uk/get-involved/meetings-in-public/technology-appraisal-committee. Accessed: September 2, 2024.
- Ministerio de Sanidad, “Plan para la consolidación de los informes de posicionamiento terapéutico de los medicamentos en el sistema nacional de salud,” 8 August 2020, Available at: https://www.sanidad.gob.es/areas/farmacia/infoMedicamentos/IPT/docs/20200708.Plan_de_accion_para_la_consolidacion_de_los_IPT.actCPF8Julio.pdf. Accessed: September 2, 2024.
- IQVIA, “EFPIA Patients W.A.I.T. Indicator 2023 Survey,” June 2024. Available at: http://www.farmaindustria.es/web/wp-content/uploads/sites/2/2024/06/EFPIA-Patient-W.A.I.T.-Indicator-Final.REV-080524.pdf. Accessed: September 2, 2024.
- Vida, J. , Oliva, J., Lobo, F., “La (des)organización de la evaluación de la eficiencia de medicamentos y otras tecnologías sanitarias en españa: diagnóstico” FUNCAS, February 2023. Available at: https://www.funcas.es/wp-content/uploads/2023/03/La-desorganizacion-de-la-evaluación.pdf. Accessed: September 2, 2024.












