When developing the health economics and outcomes research (HEOR) strategy for your product, several elements of value must be considered to optimize its reimbursement. Developing an optimal health technology assessment (HTA) strategy before pivotal trial design is particularly relevant now that payers and HTA bodies in various markets are appraising products at earlier stages of development when evidence is often limited. We present to you nine key questions to help with planning your evidence generation strategy before your pivotal trial to ensure you have a strong evidence base for your product at time of reimbursement.
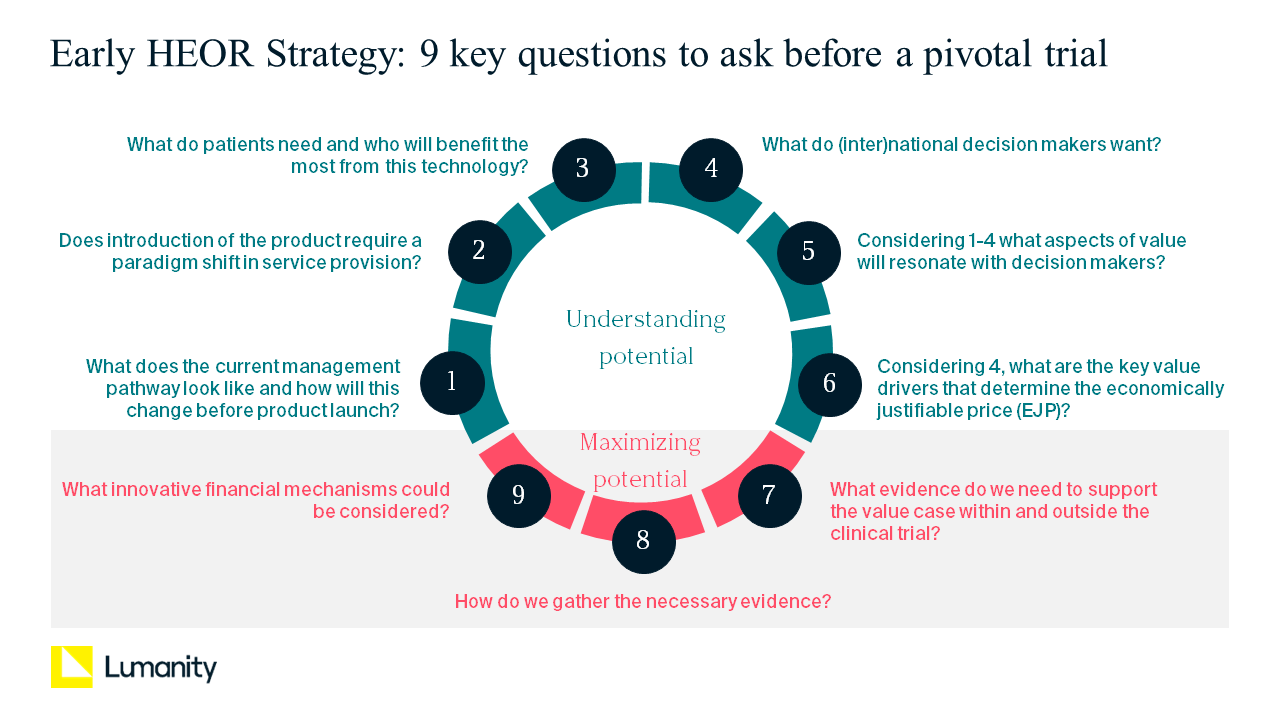
1. What will the management pathway look like at the time of product launch?
A key step in optimizing your product’s value case is to establish the current and future standard of care, identifying likely comparators and the technology’s optimal place in the treatment pathway. Considering the landscape into which your product launches is fundamental to your product’s HEOR strategy and allows you to anticipate the comparative evidence requirements that will be needed when your product is being assessed at HTA.
Recommended activities:
- Current and future country-specific treatment landscaping/literature review
- Clinical expert interviews/advisory board
2. What will patients need, and who will benefit the most from this technology?
Engaging with patients to understand the true burden of illness and how your product will meet patients’ needs is critical for success at HTA. Considering specific subgroups of patients who are likely to benefit most from the technology allows you to focus your evidence generation, quantify the size of the target population, and realize the potential for ‘optimized access’.
Recommended activities:
- Burden of illness research (qualitative interviews, patient preference studies, literature reviews)
- Publications on unmet need
3. Does introducing the product require a major change in service provision? If so, how can that be facilitated?
Understanding what would be needed for the healthcare service to deliver the technology to patients, and whether it will change the flow of patients or the expertise needed, is critical in understanding your product’s value. Specifically, changes related to diagnosis, administration (in a specialist or general setting?), monitoring, and education should be considered.
Recommended activities:
- Healthcare expert and payer interviews
4. What evidence do (inter)national decision makers want?
Understanding both the clinical and economic assessment requirements in the key markets of interest and how previous treatments in the disease area were perceived by regulators, HTA agencies, and payers allows you to develop an evidence generation strategy that meets the needs of decision makers. Understand how best to present evidence to lead to optimal decision making.
Note: consider the EU network for joint clinical assessment.
Recommended activities:
- HTA and payer landscaping (processes and methods, review of related appraisals, etc.)
- Early engagement with decision makers (advisory boards, scientific advice)
5. What is the aspirational payer value proposition for your product?
Integrating the information on patient unmet need, the expected benefits of the product, and key decision drivers in developing an early value story allows you to consider how your product addresses a priority need (e.g. severity, rarity) in the key market(s) of interest, as well as the clinical, economic, and humanistic value drivers.
Recommended activities:
- Aspirational value proposition
- Testing value proposition with clinical and payer experts
6. What are the key value drivers that determine the economically justifiable price (EJP)?
At this stage, you will need to consider the most appropriate modelling approach that best captures the value of your product. The use of early modelling allows you to explore EJP and the levers that influence this (e.g. threshold analysis/pressure testing of key clinical inputs versus expected results). Ultimately, understanding the key drivers of economic value at an early stage of development gives you time to collate and strengthen evidence in these key areas.
Recommended activities:
- Early economic modelling
- Payer engagement to support early modelling
- Clinical engagement to confirm modelling inputs
7. What evidence (within and beyond the clinical trial) is needed to support the aspirational value proposition?
It is fundamental to consider whether clinical trial endpoints are likely to capture the value of your product in terms of efficacy, safety, health-related quality of life (HRQL), and cost and also to consider how the required evidence can be best captured outside the trial design. Carrying out a deep dive into clinical trial design and an evidence generation programme and applying knowledge of key markets (Question 4) and your value case (Question 5) will allow for adjustments to the clinical trial design and identification of priority areas for further research.
Recommended activities:
- Evidence assessment and gap analysis
8. What are the optimal approaches to filling the key evidence gaps in the available time?
Considering the HEOR strategy prior to pivotal trial design lends time to develop and action an optimal evidence generation plan. Consider the most robust, payer-preferred approaches to evidence generation in the key markets of interest (Question 4), for example: systematic literature reviews, surrogacy validation, indirect treatment comparisons and expert elicitation. Being pragmatic is essential, consider what is achievable in the available time.
Recommended activities:
- Strategic evidence generation planning and execution for key markets of interest
9. What innovative financial mechanisms could be considered?
HTA and reimbursement decision makers are increasingly open to risk-sharing agreements and coverage with evidence development, especially for high-cost drugs and/or where there is greater uncertainty or unmet need. Specific funds (e.g. Innovative Medicines Fund in the UK, 5% Fund in Italy) are often available for certain types of technologies, depending on their innovativeness and indication.
Recommended activities:
- Market access and pricing strategy and strategic HTA support throughout the process
How we can help
Lumanity is committed to improving patient health by accelerating and optimizing access to innovative, life-improving medications. We have worked in delivering high-quality HTA submissions for our clients for over 15 years and therefore we understand how best to create the optimal value case for reimbursement. We can support your HEOR needs from the early stages of your clinical trial programme by developing a compelling early HEOR strategy plan and executing the plan through to successful reimbursement while providing strategic support throughout the process. We will help you synthesize the evidence needed for high-impact HEOR strategy and HTA submissions and negotiations and ensure that you have the best value case for your product. For more information, please contact us.
Contact us
If you would like to find how we can help you develop your early HEOR strategy, please contact us.











