Authored by BresMed, now part of Lumanity
As the UK continues to define a new regulatory and access environment post-Brexit, the National Institute for Health and Care Excellence (NICE) is undertaking a timely review of the methods of health technology evaluation.1, 2
This HTA methods review applies across all four of NICE’s technology evaluation programs – technology appraisals, highly specialized technologies, medical technologies and diagnostics – and is linked to the commitments in the 2019 Voluntary Scheme for Branded Medicines Pricing and Access. The scheme specifies that NICE’s cost-effectiveness thresholds will not change and that changes to the methods will respond to new types of innovation and be consistent with improving the health gain achieved by spending on new innovative medicines.
At the start of the consultation NICE noted that any changes to the methods should:
- Support patients and the NHS in accessing clinically and cost-effective health technologies
- Align with changing UK regulatory systems (particularly the Medicines and Healthcare products Regulatory Agency [MHRA])
- Support the attractiveness of the UK as a first-launch country for important and promising new health technologies, to ensure that people can access them as early as possible
- Facilitate equitable consideration of commercial and managed access flexibilities, and recognize and respond to experience with the Cancer Drugs Fund and other commercial and managed access arrangements
- Enable the technological change and innovation that the UK wants to encourage and support, by ensuring fair, robust and predictable evaluations across new and existing technology types
To assist the consultation NICE has released some details about reviewing its methods ‘to remain at the cutting edge’. This information is not as detailed as we would have liked but already we can identify some foreshadowing of future evidence requirements. We expect the consultation to consider the timeframe required for manufacturers to adjust to the new requirements – particularly where evidence generation is required. We believe it must also consider the time and resource burden needed to address the multiple analyses specified within the Decision Support Unit’s technical support documents. This is already an issue for partitioned survival analysis versus state transition modelling post the release of TSD19.3 We hope that pragmatic decisions will be made regarding when such analyses are likely to be informative and when they are likely to be superfluous.
The NICE methods review for health technology evaluation has two stages:
Stage 1: To make the case for change. Evidence from five sections of potential cases for change are being considered:
- Valuing the benefits of health technologies
- Understanding and improving the evidence base
- Structured decision making
- Challenging technologies, conditions and evaluations
- Aligning methods across programmes
Stage 2: To add detail. NICE will consider stakeholders’ responses to the case for change alongside the wider implications of any amendments. It will then develop a structured decision-making framework. These changes will be included in an updated programme manual. Once this stage is complete NICE will use the new methods in its health technology evaluations.
The next stages in the consultation
NICE’s consultation on Stage 1 will close on 18 December. To participate, see here. This form provides space to respond to the consultation questions for each area and is quite prescriptive in the areas NICE is looking for input on, but does allow for additional comments.
In February, NICE will launch a 6-week consultation on the processes for evaluation. A draft programme manual including Stage 2 of the methods review will be published next June for consultation. The final manual is scheduled to be published in September 2021 and its recommendations are due to be implemented in October.
How we expect changes to impact manufacturers
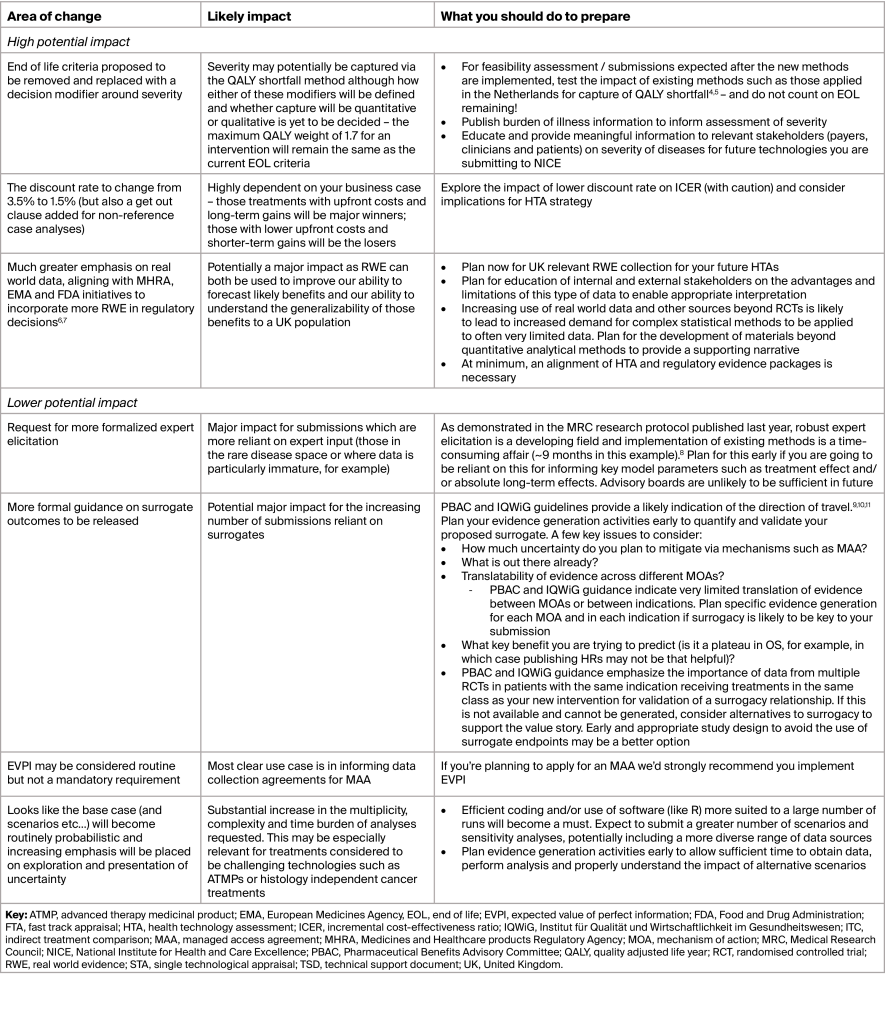
NICE’s consultation is focused on change which is likely to be significant in some areas. Table 1 outlines the most significant areas of expected change, the likely impact for manufacturers, and our opinions on what you can do now to prepare for these changes.
We will continue to monitor this process as it develops and welcome the opportunity to discuss it in more detail with you. Please contact us for more information.
Table 1: Key changes and how you can prepare for them
References
- National Institute for Health and Care Excellence (NICE). Reviewing our methods for health technology evaluation: consultation. 2020. Available at: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/chte-methods-consultation. Accessed: 25 November 2020
- Sculpher M, Soares M, Teljeur C and Watson I. Methodological and Normative Issues Arising in Recently Updated National Guidelines for Economic Evaluation. Virtual ISPOR Europe 2020. 16-19 November 2020. Workshop W2.
- Woods B, Sideris E, Palmer S, et al. NICE DSU Technical Support Document 19: Partitioned survival analysis for decision modelling in health care: A critical review. 2017. Available at: http://nicedsu.org.uk/technical-support-documents/partitioned-survival-analysis-tsd/. Accessed: 26 November 2020.
- Reckers-Droog VT, van Exel NJA and Brouwer WBF. Looking back and moving forward: On the application of proportional shortfall in healthcare priority setting in the Netherlands. Health Policy. 2018; 122(6):621-9.
- Zorginstituut Nederland (ZIN). Rapport kosteneffectiviteit in de praktijk.
2015. Available at: https://www.zorginstituutnederland.nl/binaries/zinl/documenten/
rapport/2015/06/26/kosteneffectiviteit-in-de-praktijk/Kosteneffectiviteit+in+de+praktijk.pdf. Accessed: 14 October 2019. - Medicines and Healthcare products Regulatory Agency (MHRA). MHRA draft guidance on randomised controlled trials generating real-world evidence to support regulatory decisions 2020. Available at: https://www.gov.uk/government/consultations/mhra-draft-guidance-on-randomised-controlled-trials-generating-real-world-evidence-to-support-regulatory-decisions. Accessed: 25 November 2020.
- Sharma V. EMA Consults On Using Registry Studies As RWE Source. 2020. Available at: https://pink.pharmaintelligence.informa.com/PS143000/EMA-Consults-On-Using-Registry-Studies-As-RWE-Source. Accessed: 25 November 2020.
- Bojke L, Soares MFO, Fox A, et al. Developing a reference protocol for expert elicitation in healthcare decision making. Health Technology Assessment Reports (In Press). 2019.
- Ciani O, Buyse M, Drummond M, et al. Use of surrogate end points in healthcare policy: a proposal for adoption of a validation framework. Nature Reviews Drug Discovery. 2016; 15(7):516.
- Institute for Quality and Efficiency in Health Care (IQWiG). Validity of surrogate endpoints in oncology. 2011. Available at: https://www.iqwig.de/download/A10-05_Executive_Summary_v1-1_Surrogate_endpoints_in_oncology.pdf. Accessed: 25 November 2020.
- Pharmaceutical Benefits Advisory Committee (PBAC). Report of the surrogate to final outcome working group to the Pharmaceutical Benefits Advisory Committee: a framework for evaluating proposed surrogate measures and their use in submissions to PBAC. 2008. Available at: http://www.pbs.gov.au/industry/useful-resources/pbac-technical-working-groups-archive/surrogate-to-final-outcomes-working-group-report-2008.pdf. Accessed: 25 November 2020.
- Asaria M, Griffin S and Cookson R. Measuring Health Inequality in the Context of Cost-Effectiveness Analysis. 2013. Available at: https://www.york.ac.uk/media/che/documents/Measuring%20health%20inequality%20in%20the%20context%20of%20cost-effectiveness%20analysis.pdf. Accessed: 25 November 2020.
- McNamara S, Holmes J, Stevely AK and Tsuchiya A. How averse are the UK general public to inequalities in health between socioeconomic groups? A systematic review. The European Journal of Health Economics. 2020; 21(2):275-85.











