Authored by BresMed, now part of Lumanity
The first chimeric antigen receptor (CAR) T-cell therapies launched 3 years ago, promising dramatic benefits. Patients now await the second cluster – including the novel technologies KTE-X19, lisocabtagene maraleucel, idecabtagene vicleucel and ciltacabtagene autoleucel – alongside the launch of KYMRIAH® and YESCARTA® into earlier lines of therapy.1 As consultants engaged with the first, we remember the vista of uncertainty: how do we robustly demonstrate to health technology assessment (HTA) bodies the expected value of these expensive interventions with only short-term, single-arm data as supportive evidence, the kind of data such bodies are typically so skeptical of? To paraphrase a team member, we were to ‘go where no one has gone before’.a As the second cluster approaches launch, we can claim no such excuse. Join us as we learn from past reviews from the Institute for Clinical and Economic Review (ICER) and the National Institute for Health and Care Excellence (NICE), draw four implications for the future, and then pose four further questions for your consideration.
First, a summary of our emerging context: a necessity if we are to rigorously draw out future implications from the recent CAR T-cell HTA history.
[a] For full transparency we must admit that, unfortunately, the team member was not Captain Kirk
The emergence of CAR T-cell therapies and the evolving HTA landscape
CAR T-cell technology undoubtedly represents a remarkable step change in care for life-limiting diseases previously considered incurable. In 2017, the launch of the first CAR T-cell therapy prompted the Food and Drug Administration (FDA) commissioner to announce that ‘we’re entering a new frontier in medical innovation with the ability to reprogram a patient’s own cells to attack a deadly cancer’.2 In 2018, the curative potential of these transformative therapies led to them being declared the advance of the year by the American Society of Clinical Oncology (ASCO) in its annual report.3 So far, CAR T-cell treatments have launched in severe, end-of-treatment-line haematological malignancies where, at least in earlier lines of treatment, there has been ‘intent to cure’ with the current standard of care. They have therefore received the tail winds of both a high unmet need and an openness to their curative potential. This urgency, combined with the impressive outcomes generated in clinical trials, has led to accelerated regulatory approval by the FDA and the European Medicines Agency (EMA) based on single-arm trials of limited duration. Few would question the regulators’ decision that these technologies have a positive benefit–risk profile.4-7 Of course, the short-term, single-arm data are problematic for HTA. To robustly assess value, it is necessary to not only reach a judgement concerning the probable existence of an incremental health benefit, but also to reach one on its magnitude. Nevertheless, HTA bodies have so far allowed patient access, typically adopting some combination of advanced analytic methods, outcome-based agreements (OBAs), and further evidence generation agreements to characterize and share the risk burden of the uncertainty between promise and realization.
Transformation in medical technology is necessitating an evolution in routine technology appraisal methods. Since the launch of the first CAR T-cell therapies, ICER has published their ‘valuing a cure’ white paper: ‘Adapted Value Assessment Methods for High-Impact “Single and Short-Term Therapies” (SSTs)’.8 For these transformative therapies, ICER will 1) make cure proportion modelling (e.g. mixture-cure models) the standard reference case (as traditional parametric curves may not adequately fit the survival data, owing to the possibility that a proportion of the population is cured); and 2) consider a value modifier that accounts for the possibility that patients may place an additional value on long-term survival, above the associated benefits in life years (LYs) and quality-adjusted life years (QALYs). Similarly, NICE is currently updating their methods guide for assessing new technologies, in part because of the launch of cell and gene therapies.9 Like ICER, NICE’s guidance considers which modification factors should be incorporated when assessing a technology’s value, and how the uncertainty surrounding a technology’s extrapolated treatment benefit should be characterized.
While there are undoubtedly lessons to learn from prior assessments, both the field of CAR T-cell therapy and the associated methods of HTA are undergoing change. How should manufacturers learn and adapt, as their CAR T-cell therapies launch in indications in which 1) the unmet need is not as substantial (e.g. if launching in an earlier line of therapy); 2) the plausibility of ‘curative potential’ is reduced (e.g. if historically the paradigm for the therapy area has been one of maintaining control rather than ‘cure’)10; and 3) the magnitude of incremental survival benefit offered is only substantive, rather than superlative?b
In this paper, we focus on how manufacturers can best position their initial HTA submissions for soon-to-launch CAR T-cell therapies.c We draw inference from prior assessments by ICER and NICE, while being mindful of the changes that ICER has made since the ‘valuing a cure’ white paper was announced8 and those that NICE is currently undertaking.9
[b] Either because the current standard of care, or a novel therapy that is not a CAR T-cell therapy, is relatively effective (for example, bispecific antibodies); patients progressing on the current standard of care will receive a currently launched CAR T-cell therapy in a later line; another CAR T-cell therapy as a relevant comparator; or the existence of a ‘cure’ is not considered plausible.
[c] We were tempted to include musings on the crucial role of OBAs, subsequent evidence generation and the future impact of autologous CAR T-cell therapies. However, the paper would have become like the first author’s thesis: it would have only been read by the author(s) and reviewers.
Lessons from ICER and NICE assessments of the ‘first cluster’
Table 1: Preferred approaches of ICER and NICE to areas of importance when assessing value
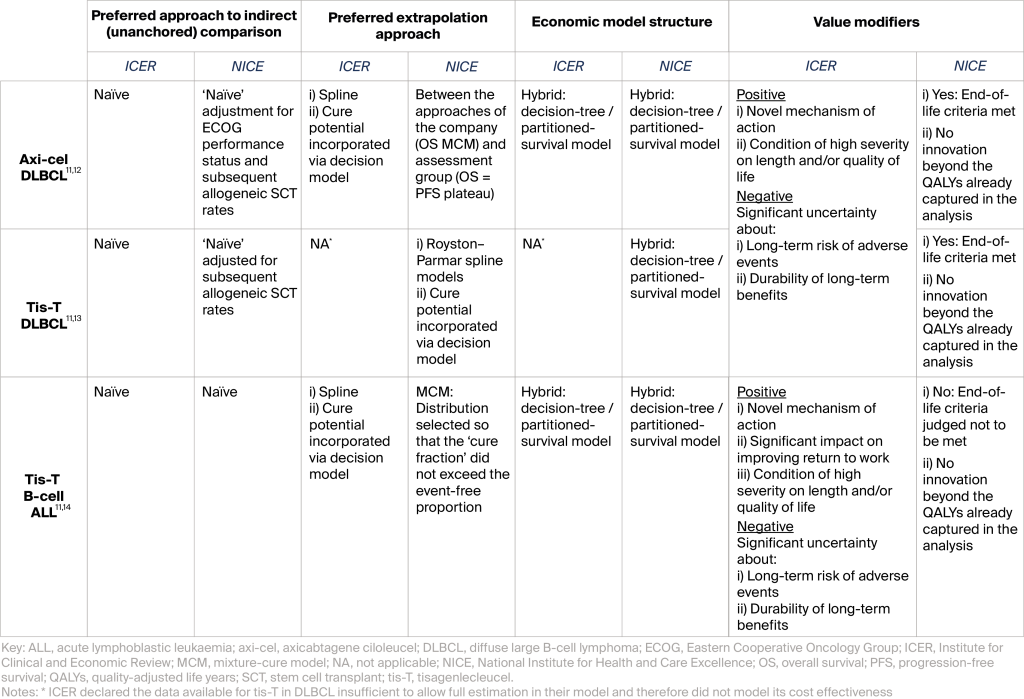
In assessments of CAR T-cell treatments to date, two topics have been of greatest importance when assessing value: drawing reliable estimates of relative treatment benefit with non-randomized data (‘unanchored’ indirect treatment comparisons), and robustly estimating long-term survival from short-term trial data (extrapolations). Table 1 (above) summarizes the preferred approaches of ICER and NICE to these critical areas, alongside: 1) the model structure selected to synthesize these analytics with their associated survival, QALY, and cost consequences; and 2) the modifiers used to ‘up-lift’ or ‘down-weight’ the value of the resulting cost-effectiveness estimates.
Approaching the future assessments
1. Estimating relative treatment effect
Given the lack of viable treatment options in later-line B-cell acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL), CAR T-cell therapies have so far been approved based on single-arm trial data. Interestingly, ICER and NICE have preferred to rely on naïve or close-to-naïve relative-effectiveness comparisons, despite the existence of many established analytic methods to adjust for imbalances across the observed characteristics of the intervention trial data and the comparator trial data (e.g. matching-adjusted indirect comparison or simulated treatment comparison when only population-level comparator data are available, and a further suite of approaches for when patient-level data are available across treatment arms). ICER and NICE have endorsed analyses that, at most: 1) undertake simple adjustments on the comparator data by only including patients assessed to have a good Eastern Cooperative Oncology Group performance status; and 2) re-weight the overall survival (OS) outcomes according to the assumed proportion of patients who went on to receive allogeneic stem cell transplant. In part, this is likely because although the more statistically minded assessors may prefer the more advanced methods in principle, they nevertheless must acknowledge that it is impossible to truly eliminate the potential bias from unobserved differences in the cohorts included in each data source where there is no common comparator. Clinical plausibility of the estimated comparator outcomes has therefore reigned supreme; KISS has been the operative acronym.
As CAR T-cell therapies launch in earlier treatment lines, they are mandated to be assessed through randomized controlled trials. In these contexts, there are viable treatment options against which regulators require robust evidence of superiority. To date, in end-of-line contexts, HTA bodies have – out of necessity – accepted single-arm trial data. As a result, ‘CAR T-cell therapy’ and ‘single-arm trial evidence’ are currently subliminally linked concepts. Once this subliminal link is broken, even when launching in end-of-line contexts, will HTA bodies be increasingly sceptical of relative-effectiveness estimates derived from non-randomized data?
2. Estimating lifetime overall survival benefit from trials with limited follow-up
To date, ICER and NICE have been open to extrapolation approaches that enable a proportion of patients to have estimated survival close to the equivalent age-matched general population. This is perhaps unsurprising, given the mechanism of action of CAR T-cell therapies – with ‘cure’ as an ambition within the current B-cell ALL and DLBCL treatment paradigms as well as the observed trial data to date. ICER’s willingness to accept such assumptions seems to be increasing in general (the ‘valuing a cure’ white paper endorses a reference case using cure modelling), though in specific indications ICER tends to be more cautious. In the draft scope for CAR T-cell therapies in relapsed or refractory multiple myeloma, ICER both highlights that cure has not to date been a realizable treatment objective and questions whether the assessment should be undertaken under the SST framework, noting that the current evidence suggests that ‘…CAR-T therapies extend progression-free survival by a magnitude that is similar to previously-approved therapies’.10 What is the broader implication of this draft scope and the endorsed approaches to date? Any argument to ‘cure’ (or the presence of some long-term survivors) needs to be anchored in the specific nature of the indication and treatment lines. Additionally, when selecting their base case estimate for the ‘cure fraction’ (or similar), the manufacturer should select one that aligns with clinically related indicators .
As CAR T-cell therapies begin to move into earlier treatment lines within DLBCL and subsequently relapsed or refractory multiple myeloma, it will become harder to demonstrate the dramatic relative OS benefits that HTA bodies have to date associated with CAR T-cell therapy. OS benefit consists of a strategy’s survival prior to and following progression – referred to as progression-free survival (PFS) and post-progression survival (PPS), respectively. As CAR T-cell therapies launch in earlier lines, they may face the challenge of:
1. An effective comparator treatment in the same line of therapy where the CAR T-cell therapy’s relative performance in PFS and consequently OS is less impressive than versus palliative options in the current end of line indications; and/or
2. Patients on the comparator treatment strategy progressing onto a highly effective CAR T-cell therapy in later line, so that the comparator treatment strategy would then have improved PPS and consequently OS
The challenge may be compounded if the distribution of subsequent therapies within the typically US-based trials differs from that seen in the jurisdiction in which the new CAR T-cell therapy is being assessed. In earlier lines, how can manufacturers demonstrate substantial therapeutic value if the OS gain appears limited?
3. Integrating the relative clinical, health-related quality-of-life, and cost outcomes for the CAR T-cell therapy versus current standard of care
For the assessments so far, partitioned-survival models – the default structure of choice in oncology HTA – have been deemed suitable. Minor modifications have been incorporated to capture divergent outcomes by responders and non-responders in the ICER assessments. Similarly, in the NICE assessments, an initial decision tree has been explored to incorporate those few patients intended for treatment with CAR T-cell therapy who then did not receive the infusion. Still, the core of the model follows the partitioned survival approach, which transparently links prespecified clinical trial endpoint data (OS and PFS) to patient health states, while easily allowing results from advanced extrapolation approaches such as cure models for OS to be incorporated within the structure. If a manufacturer is launching a CAR T-cell therapy in an end-of-line treatment indication, the model structure of choice remains a partitioned-survival model.d However, the choice may not be as simple if the CAR T-cell therapy is launched in an earlier treatment line, where patients may still progress onto highly effective subsequent therapies.
Could state-transition models have an important role to play in earlier-line CAR T-cell indications? Similar to the use of immune-checkpoint inhibitors in the earlier adjuvant 15-17, a state-transition approach would be particularly advantageous if the proportion of subsequent treatments received in the trial’s comparator arm is likely to differ from that seen in routine treatment practice – e.g. if ‘too many’ patients progress onto an alternative CAR T-cell therapy in the trial.18 This would allow the manufacturer to model time to progression using within-trial data; then, drawing on both within-trial data and external sources, the manufacturer could model time from progression to death, stratified by different initial treatments, and consider the types of subsequent treatments received. This extra flexibility would allow the manufacturer to explicitly: 1) identify how highly efficacious subsequent therapies are ‘confounding’ the observed survival benefit in the trial (though this relationship may be genuine if such therapies are used in routine practice); and 2) re-calibrate expected comparator survival outcomes and costs if the distribution of subsequent therapies used in routine practice differs from that seen in the trial.e
[d] Apologies to our more technical readers, for whom this is likely stating the obvious!
[e] Of course, an alternative approach to this situation is to use crossover adjustment techniques such as the two-stage, IPCW and RPSFT approaches. If such methods are appropriate and feasible, then crossover-adjusted comparator OS can be incorporated into the partitioned survival modelling framework.
4. Contextualizing the cost-effectiveness estimates within the value modifiers of relevance
To paraphrase a line from George Orwell’s famous allegory: even in HTA systems focused on cost effectiveness, ‘some QALYs are more equal than others’. For the reimbursement of CAR T-cell therapies, the importance of explicit and implicit value modifiers is clear. In the DLBCL CAR T-cell appraisals, it could be argued that because of implicit value modifiers, NICE – often seen as among the most transparent and objective of the HTA bodies globally – was straining every sinew to conclude that the end-of-life criteria were met. Without the higher threshold of ~£50,000/QALY gained being granted, the price required to achieve cost effectiveness may have been unviable for the manufacturer, and the English healthcare system would have been unable to access transformative therapies it seemingly values above its explicit thresholds and stated modification factors.
The healthcare systems in the US and in England are very different from one another, and ICER does not have the same reimbursement decision-making authority as NICE. Across markets, these factors have accentuated the enthusiasm around the potential dramatic health benefits offered by CAR T-cell therapies, generating sufficient demand to offset uncertainty around the favourable assumptions needed to justify their high price. Given the HTA challenges referred to in the introduction, it is strategically critical that manufacturers carefully communicate evidence-based value messages that address HTA bodies’ implicit and explicit value modifiers, throughout submission materials.
Many of the future clusters of CAR T-cell therapy launches will be in earlier lines of therapy where the unmet need is less stark, and the additional survival benefit may not be as dramatic. Additionally, excitement around the currently novel mechanism of action may begin to diminish, even with advances in subsequent generations of CAR T-cell therapy. In indications with less favourable value modifiers, how can manufacturers achieve the cost flexibility required to ensure patient access?
Our recommendations given the current emerging context, and further considerations we expect to come into play as the assessment experience matures
1
Focus on the plausibility of estimated clinical outcomes of your comparator
As some CAR T-cell therapies begin to be approved based on randomized controlled trial data, will HTA bodies be increasingly sceptical of non-randomized data, even for CAR T-cell therapies launched in end-of-line contexts?
2
Anchor inferences about the proportion ‘cured’ in the wider clinical context (plausibility of cure in the indication/line; observed trial proportion in durable complete/deep response)
In earlier lines, how can manufacturers demonstrate substantial therapeutic value if the OS gain appears limited?
3
If launching a CAR T-cell therapy in an end-of-line indication, choose a partitioned-survival model structure
Could state-transition models have an important role to play in earlier-line CAR T-cell therapy indications if subsequent treatments differ between trial and clinical practice?
4
Carefully communicate evidence-based value messages that address HTA bodies’ implicit and explicit value modifiers, throughout submission materials
In indications with less favourable value modifiers, how can manufacturers achieve the cost flexibility required to ensure patient access?
References
- Blue Matter Consulting. Cell-Based Therapies: 2019 Year in Review and Upcoming Milestones. 2020. Available at: https://bluematterconsulting.com/cell-based-therapies-2019-review-upcoming-milestones/. Accessed: 20 October 2020.
- Food and Drug Administration (FDA). FDA approval brings first gene therapy to the United States. 2017. Available at: https://www.fda.gov/news-events/press-announcements/fda-approval-brings-first-gene-therapy-united-states. Accessed: 21 October 2020.
- American Society of Clinical Oncology (ASCO). CAR T-Cell Immunotherapy Named Advance of the Year in Annual ASCO Report. 2018. Available at: https://www.asco.org/about-asco/press-center/news-releases/car-t-cell-immunotherapy-named-advance-year-annual-asco-report. Accessed: 22 October 2020.
- U.S. Food and Drug Administration (FDA). KYMRIAH (tisagenlecleucel). (Updated: 28 March 2019) Available at: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel. Accessed: 20 October 2020.
- U.S. Food and Drug Administration (FDA). YESCARTA (axicabtagene ciloleucel). (Updated: 28 May 2020) Available at: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta-axicabtagene-ciloleucel. Accessed: 20 October 2020.
- European Medicines Agency (EMA). Kymriah. (Updated: 17 August 2020) Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah.
- European Medicines Agency (EMA). Yescarta. (Updated: 26 June 2020) Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/yescarta. Accessed: 20 October 2020.
- Institute for Clinical and Economic Review (ICER). Adapted Value Assessment Methods for High-Impact “Single and Short-Term Therapies” (SSTs). 2019. Available at: https://icer-review.org/wp-content/uploads/2019/01/ICER_SST_FinalAdaptations_111219.pdf. Accessed: 20 October 2020.
- National Institute for Health and Care Excellence (NICE). NICE announces details of health technology evaluation methods review. 2019. Available at: https://www.nice.org.uk/news/article/nice-announces-details-of-health-technology-evaluation-methods-review. Accessed: 20 October 2020.
- Institute for Clinical and Economic Review (ICER). Anti B-Cell Maturation Antigen CAR T-cell and Antibody Drug Conjugate Therapy for Triple Class Refractory Multiple Myeloma. Draft Background and Scope. 2020. Available at: https://icer-review.org/wp-content/uploads/2020/08/ICER_Multiple-Myeloma_Draft-Scope_092220.pdf. Accessed: 20 October 2020.
- Institute for Clinical and Economic Review (ICER). CAR-T Therapies: Final Evidence Report. 2018. Available at: https://icer-review.org/material/car-t-final-report/. Accessed: 20 October 2020.
- National Institute for Health and Care Excellence (NICE). Axicabtagene ciloleucel for treating diffuse large B-cell lymphoma and primary mediastinal large B-cell lymphoma after 2 or more systemic therapies. Technology appraisal guidance [TA559]. 2019. Available at: https://www.nice.org.uk/guidance/ta559. Accessed: 20 October 2020.
- National Institute for Health and Care Excellence (NICE). Tisagenlecleucel for treating relapsed or refractory diffuse large B-cell lymphoma after 2 or more systemic therapies. Technology appraisal guidance [TA567]. 2019. Available at: https://www.nice.org.uk/guidance/ta567. Accessed: 20 October 2020.
- National Institute for Health and Care Excellence (NICE). Tisagenlecleucel for treating relapsed or refractory B-cell acute lymphoblastic leukaemia in people aged up to 25 years. Technology appraisal guidance [TA554]. 2018. Available at: https://www.nice.org.uk/guidance/ta554. Accessed: 20 October 2020.
- National Institute for Health and Care Excellence (NICE). Nivolumab for adjuvant treatment of completely resected melanoma with lymph node involvement or metastatic disease. Technology appraisal guidance [TA558]. 2019. Available at: https://www.nice.org.uk/guidance/ta558. Accessed: 22 October 2020.
- National Institute for Health and Care Excellence (NICE). Pembrolizumab for adjuvant treatment of resected melanoma with high risk of recurrence. Technology appraisal guidance [TA553]. 2018. Available at: https://www.nice.org.uk/guidance/ta553. Accessed: 22 October 2020.
- National Institute for Health and Care Excellence (NICE). Palbociclib with fulvestrant for treating hormone receptor-positive, HER2-negative, advanced breast cancer. Technology appraisal guidance [TA619]. 2020. Available at: https://www.nice.org.uk/guidance/ta619. Accessed: 22 October 2020.
- Batteson R, Hart R, Hemstock M, et al. Modelling Survival of Patients Treated with Adjuvant Nivolumab Who Have Melanoma with Lymph Node Involvement or Metastatic Disease After Complete Resection. Pharmacoecon Open. 2020; 4(2):343-51.












