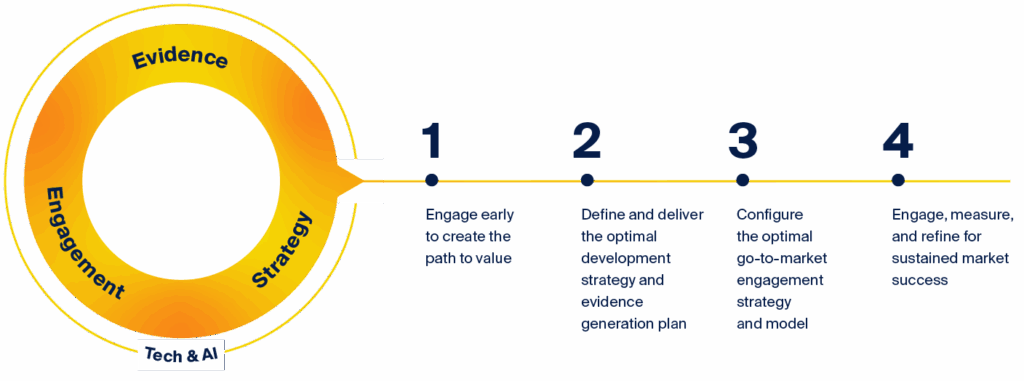
Our capabilities in strategy, evidence, engagement, and technology and AI converge to create breakthrough value. By identifying key inflection points that can significantly impact value, our experts ensure that every strategic decision contributes to a medicine’s success in reaching patients and improving their outcomes.