Authored by Clarion, now part of Lumanity
Oncology drug development has produced remarkable breakthroughs in the last decade, including various immunotherapies and precision medicine approaches, offering hope for previously incurable cancers. However, the probability of success (PoS) in oncology is among the lowest of all therapeutic areas: the likelihood of regulatory approval for programs entering Phase 1 is only 3-5%, and the likelihood of commercial success is even lower. The return on investment for R&D is at an all-time low.
How do we sustainably develop better therapies for cancer patients?
Dennis Chang, managing director at Clarion, a Lumanity business joined a group of industry leaders to discuss the challenges and opportunities for bridging the gap in oncology translation and innovation at the recent 5th Annual Emerging Frontiers in Oncology Summit. Proceeds from the summit raised funds for Life Science Cares as part of the Timmerman Traverse campaign. Dennis teamed up with Jeff Bockman to develop a presentation, titled “Oncology’s PoS Problem,” that frames the challenges of oncology translational medicine and presents relevant facts and perspectives to stimulate discussion of potential solutions.
Most analyses of drug development failure break down the reasons for failure into categories such as insufficient efficacy (the most common reason), excessive toxicity, commercial/strategic decisions by the company, failure to accrue, etc. However, we must dissect the root causes to develop potential solutions. For example, if a drug shows poor efficacy, was it due to the target biology, the drug compound, its formulation, its dose schedule, the trial population, or the trial design? Different root causes will require different solutions.
Most analyses of drug failure also focus on development/regulatory failure; we must also go beyond and consider factors behind commercial failure, such as oncology’s extraordinarily competitive market dynamics and the importance of sufficient differentiation. While technical success or failure in translational science plays a role, drugs can also fail due to a lack of distinctiveness.
Overall, we have grouped the potential solutions into two broad categories: improvements in “innovation” at the top of the drug development “funnel” and improvements in implementation to reshape the drug development funnel itself.
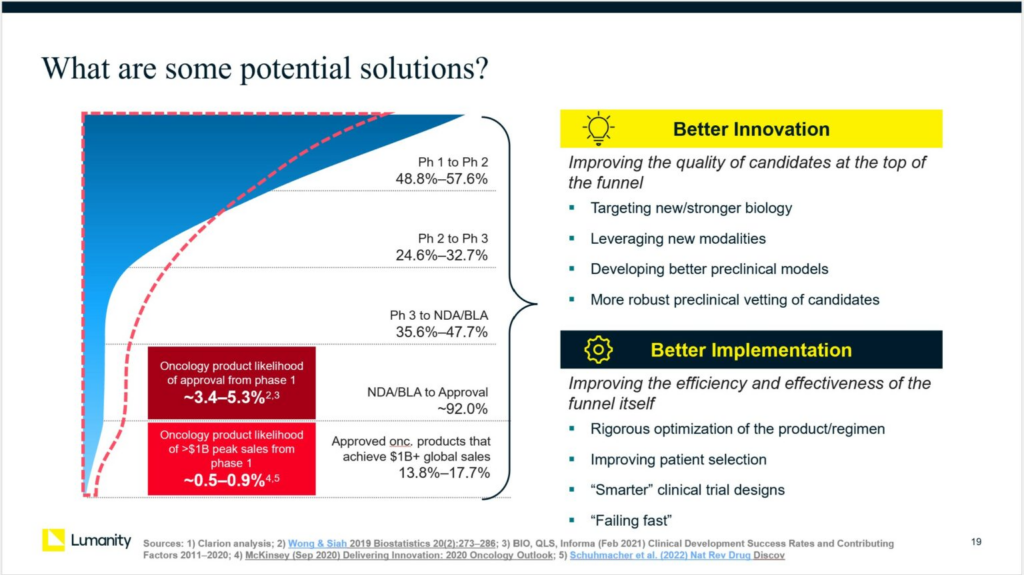
Better Innovation:
Potential approaches to improving the quality of drug candidates at the top of the “funnel” include:
- Rebalance risk and increase focus on novel targets: The biopharma pipeline exhibits a herd mentality, with hundreds of products pursuing the top 20 oncology drug targets. The first and best-in-class drugs within a class tend to capture most of the commercial value; thus if there are dozens of drugs pursuing the same target, they face significant commercial risks. Betting on novel targets incurs more technical risk, but there may be ways to mitigate that risk as our understanding of cancer biology has never been greater and our tools for interrogating that biology have never been more sophisticated. We may be able to better discern which targets/pathways have amplifying effects and which are limited by compensatory/feedback mechanisms. The pipeline (and the companies and investors behind it) must always balance technical and commercial risks, but it may be time to shift that balance toward novel targets.
- Leverage new modalities: As discussed last year, new platform technologies may enable more effective modulation of novel targets. However, the industry should be careful not to overinvest in too many novel modalities given that history shows many may be “dead ends” or require substantial further innovations before becoming competitive.
- Develop better preclinical models: Preclinical models in oncology are notoriously unreliable, especially mouse models for immuno-oncology. Immune phenotypes are so complex and variable that they are difficult to reproduce even when seeding the same mouse with the same tumor cells in different sites in the skin. Development of more predictive models would clearly be invaluable—albeit technically challenging.
- Vet preclinical candidates more robustly: At the same time, we should make better use of the models we already have. For example, we may use more stringent thresholds for positive signals, and we may use multiple kinds of models with different, complementary advantages and disadvantages.
Better Implementation:
Potential approaches to improving the effectiveness/efficiency of the “funnel” itself include:
- Optimize the product and/or regimen: Many products fail in clinical development even though the target is well validated, due to poor “drug-like properties”. Mitigating the risk of such failures may be achieved by PK/PD optimization through more rigorous attention to formulation development and/or dose schedule design.
- Improve patient selection: Precision medicine trials are known to have a better PoS than an all-comers approach by enriching for patients most likely to benefit from the therapy. Investing in biomarker development and other biology-based methods for patient selection should improve clinical success rates.
- Explore “smarter” clinical trial designs: The key trade-off for the points above is the time required to optimize regimens and to develop biomarker tests. Faster, more efficient clinical trials would be needed to offset those requirements. One concept to consider is neoadjuvant translational trials, or window-of-opportunity trials, to better understand the biological impact of a novel therapy in a fast study with a small cohort. Expanded use of adaptive trials and model-assisted designs would also improve trial efficiency.
- Improve patient accrual: Finally, we can improve trial completion rates by addressing accrual issues. Some potential approaches include increasing trial awareness, enhancing patient engagement, reducing logistical burdens, and leveraging technology for improved trial accessibility.
In the end the path forward will no doubt involve a mix of solutions laid out above, across both better innovation and better implementation. For more details, access the full presentation.